Volume 13, Issue 2 (2025)
Health Educ Health Promot 2025, 13(2): 195-204 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Istanti Y, Adnani H. Individual and Environmental Determinants of Leptospirosis in Bantul, Indonesia. Health Educ Health Promot 2025; 13 (2) :195-204
URL: http://hehp.modares.ac.ir/article-5-79776-en.html
URL: http://hehp.modares.ac.ir/article-5-79776-en.html
Yuni Istanti1, H. Adnani *1
1- Department of Public Health, STIKES Surya Global, Yogyakarta, Indonesia
Keywords: Leptospirosis [MeSH], Indonesia [MeSH], Zoonotic Disease [MeSH], Risk Factors [MeSH], Public Health [MeSH]
Full-Text [PDF 627 kb]
(514 Downloads)
| Abstract (HTML) (1412 Views)
Full-Text: (62 Views)
Introduction
Leptospirosis is a significant zoonotic disease with a global distribution, particularly affecting tropical, subtropical, and temperate regions. The disease is prevalent in areas such as the Caribbean, Latin America, the Indian subcontinent, Southeast Asia, Oceania, and parts of Eastern Europe [1]. Globalization and international travel have also increased exposure to leptospirosis in developed countries, expanding its epidemiological footprint beyond traditional endemic zones [1]. The Asia-Pacific region is notably affected, with outbreaks often linked to poor sanitation, overcrowding, and climatic conditions that favor bacterial persistence and transmission [2]. These demographic and environmental determinants create complex transmission dynamics that challenge public health control efforts across diverse geographical settings.
Leptospirosis is often underdiagnosed, leading to an underestimation of its global burden. The World Health Organization (WHO) has established the Leptospirosis Burden Epidemiology Reference Group (LERG) to estimate the global burden using disability-adjusted life years (DALYs) as a standardized metric [3]. Current epidemiological data indicate an estimated annual incidence of approximately half a million severe human cases globally; however, this figure likely represents substantial underreporting due to diagnostic limitations and surveillance inadequacies [4]. Mortality rates demonstrate significant variation, with higher rates documented in older patients and those presenting with severe clinical manifestations such as jaundice and renal failure [5]. The burden of disease extends beyond acute mortality, with persistent post-infection sequelae, including fatigue and myalgia, contributing to long-term morbidity and reduced quality of life [6].
Leptospirosis is caused by spirochetes of the genus Leptospira, with pathophysiological mechanisms that produce a remarkably diverse clinical spectrum. Disease presentations range from mild febrile illness to severe complications, including hepatorenal dysfunction, myocarditis, pulmonary hemorrhage, and meningitis [7]. The pathophysiology involves bacterial entry through mucous membranes or skin abrasions, often due to exposure to contaminated water sources, followed by hematogenous dissemination that affects multiple organ systems [7]. This complex pathophysiological profile creates diagnostic challenges and contributes to the underestimation of the disease burden, particularly in regions with limited laboratory capacity. The interplay between bacterial virulence factors, host immune responses, and environmental exposures creates a multifaceted disease entity that requires integrated surveillance and management approaches across the healthcare continuum.
Previous systematic reviews indicate that leptospirosis carries substantial morbidity and mortality metrics; however, it remains underrepresented in global health prioritization frameworks, resulting in fragmented surveillance systems and limited intervention development [8, 9]. The disease exhibits pronounced socioeconomic stratification patterns, with the highest incidence documented among marginalized communities where inadequate sanitation infrastructure, occupational exposures, and limited healthcare access create synergistic risk profiles [10-12]. Epidemiological analyses demonstrate that agricultural workers, fishermen, sewage workers, and inhabitants of informal settlements face exponentially higher exposure probabilities, reflecting the intersection between environmental determinants and structural inequalities in disease distribution [10, 12].
The persistent neglect and substantial underreporting of leptospirosis present formidable challenges to accurate burden quantification, with current surveillance systems capturing only an estimated 5-15% of actual cases across endemic regions [8, 13, 14]. This surveillance inadequacy stems from multiple intersecting factors, including the non-specific clinical presentation that frequently mimics other febrile illnesses, leading to misdiagnosis and classification errors within healthcare systems [15, 16]. Diagnostic complexity represents a fundamental barrier to effective surveillance, as the disease manifests along a broad clinical spectrum ranging from mild influenza-like illness to severe manifestations, encompassing Weil’s disease with its characteristic triad of jaundice, renal failure, and hemorrhagic phenomena [9, 11, 12, 16].
Leptospirosis determinants comprise interrelated environmental and socioeconomic factors that create complex risk landscapes with significant geographical and temporal heterogeneity. Environmental parameters, including temperature, precipitation, flooding events, and humidity levels, demonstrate consistent associations with disease incidence, with multivariate analyses identifying annual mean temperature and rainfall metrics as significant predictors of transmission intensity [17-19]. Hydrological factors, particularly flood exposure, function as critical risk amplifiers (incidence rate ratios: 2.3-8.7) [20, 21], while geographical characteristics, including land cover typologies, altitudinal gradients, and proximity to water bodies, further modulate spatial distribution patterns [18, 22]. These environmental determinants interact synergistically with socioeconomic factors, with quantitative evidence establishing significant associations between the leptospirosis burden and urbanization patterns, especially in informal settlements characterized by high population density and inadequate sanitation infrastructure [20, 23].
Housing quality metrics demonstrate strong correlations with infection risk (odds ratios: 2.21-5.15 for substandard dwellings) [23], while economic vulnerability indicators, including poverty rates and educational attainment, consistently predict disease distribution through pathways involving reduced access to protective infrastructure, occupational exposures, and limited healthcare utilization [23-25]. Advanced geospatial modeling approaches that incorporate these determinants enable high-resolution risk mapping and targeted intervention deployment, with vulnerability indices constructed from composite socioeconomic indicators providing evidence-based frameworks for public health resource allocation in endemic regions [18, 19, 22, 23].
Leptospirosis cases in Indonesia have shown an increasing trend annually. In 2020, there were 1,170 cases with 106 deaths (case fatality rate (CFR) of 9.06%, significantly higher than COVID-19 mortality), while in 2021, there were 736 cases with 84 deaths (CFR 11.41%). In 2022, based on reports from 11 provinces, there were 1,408 leptospirosis cases with 139 deaths (CFR 9.87%) [26]. Leptospirosis is believed to have the most widespread distribution globally and is known in some countries as “Rat Urine Fever.” High-risk populations include those whose occupations, living environments, or lifestyles expose them to the pathogen. High transmission risk occurs among farmers, plantation workers, pet shop employees, livestock handlers, sewer workers, slaughterhouse workers, meat processors, and military personnel [27].
According to the Yogyakarta Special Region Health Office, hundreds of leptospirosis cases are distributed across all districts and cities in the region. The highest number of cases was reported in Bantul Regency, reaching 110 cases. Yogyakarta City recorded 19 cases, Kulonprogo Regency recorded 36 cases, Gunungkidul Regency reported 56 cases, and Sleman recorded 42 cases [27]. Based on data from the Bantul District Health Office, leptospirosis cases in 2022 were 141 across 17 sub-districts, with the highest distribution in Kasihan Sub-district (15 cases) and Bantul Sub-district (13 cases), followed by Sandakan and Jetis Sub-districts (12 cases each), and Imogiri, Pundong, and Sewon Sub-districts (11 cases each). Pandak Sub-district reported 10 cases, Bambanglipuro Sub-district had 9 cases, Pleret Sub-district had 7 cases, and Piyungan and Sedayu Sub-districts each reported 6 cases. Banguntapan Sub-district had 5 cases, the Kretek, Pajangan, and Sanden Sub-districts each recorded 4 cases, and Dlingo Sub-district had 2 cases [27].
According to data from the Bantul District Health Office, leptospirosis cases in 2023 totaled 155 across 17 sub-districts, with the highest distribution in Bantul Sub-district (24 cases), followed by Sewon Sub-district (18 cases), Kasihan Sub-district (17 cases), and Pundong Sub-district (16 cases). Bambanglipuro and Pandak Sub-districts each reported 12 cases, while Sedayu Sub-district had 9 cases. Imogiri, Jetis, and Sanden Sub-districts each recorded 7 cases, and Banguntapan and Kretek Sub-districts each had 5 cases. Pleret and Srandakan Sub-districts each reported 2 cases [27].
Despite extensive epidemiological documentation of the global leptospirosis burden, significant knowledge deficits persist regarding localized transmission determinants in endemic Indonesian regions, particularly in the Yogyakarta Special Region, where case fatality rates consistently exceed 9%, markedly higher than COVID-19 mortality metrics. While surveillance data demonstrate distinct spatial clustering, with the Kasihan sub-district maintaining a high incidence, analytical investigations quantifying the relative contributions of individual versus environmental risk factors within this high-transmission zone remain conspicuously absent from the current evidence base.
This study, therefore, aimed to examine the relationship between individual factors (gender, occupation, education) and biotic environmental parameters (flood history, drainage conditions, waste disposal systems) with leptospirosis incidence in the Kasihan II Bantul Public Health Center catchment area during 2022-2023, employing a case-control analytical framework to identify high-priority modifiable determinants for targeted public health interventions aligned with local epidemiological patterns and resource constraints.
Instrument and Methods
Study design
This observational analytical study employed a retrospective case-control approach to investigate the relationship between individual and environmental factors and leptospirosis incidence. This methodological framework was selected to enable a quantitative assessment of risk factor associations by comparing exposure histories between individuals with confirmed leptospirosis diagnoses and matched controls without the disease. The retrospective orientation facilitated the examination of temporal relationships between potential risk factors and disease occurrence while maximizing efficiency within the constraints of available resources and the epidemiological context.
The case-control methodology was chosen after systematic consideration of alternative designs, including prospective cohort approaches. The retrospective design offered practical advantages in the study context, including feasibility for investigating relatively uncommon outcomes within resource and temporal constraints, capacity for simultaneous evaluation of multiple exposure parameters, and established precedent in the leptospirosis epidemiological literature, facilitating cross-study comparisons. However, this approach introduced methodological limitations, including potential recall bias in exposure assessment and the inability to establish precise temporal sequences between environmental exposures and disease occurrence. These limitations were explicitly addressed in the discussion section, with appropriate interpretive caution regarding causal inference.
Population and sampling
The target population comprised all leptospirosis patients residing within the catchment area of the Kasihan II Bantul Public Health Center. A total sampling technique was implemented to recruit all eligible cases diagnosed during the 2022-2023 study period, yielding 17 confirmed leptospirosis patients. Controls were selected using a 1:1 matching ratio, with 17 individuals without leptospirosis recruited from the same geographic area, resulting in a total sample of 34 participants.
Sample size considerations included a retrospective power analysis demonstrating adequate statistical capacity (1-β=0.80, α=0.05) to detect large effect sizes (OR≥5.0) but limited power for moderate (OR=2.0-3.0) or small effect associations. This statistical parameter was deemed acceptable for primary risk factors with established high-magnitude associations based on previous epidemiological investigations, though it may be insufficient for detecting environmental determinants with more modest effect sizes. The sampling approach was designed to maximize analytical validity within the constraints of case availability in this endemic region, with case identification protocols that included a comprehensive surveillance system review to ensure the inclusion of all laboratory-confirmed infections during the study period.
Inclusion criteria for cases encompassed a laboratory-confirmed leptospirosis diagnosis through microscopic agglutination testing or PCR methodologies and current residence within the study area. Controls were selected from the same communities to ensure comparable exposure opportunities to environmental risk factors. Control recruitment employed frequency matching to maintain demographic comparability in age distribution while allowing for analytical assessment of other hypothesized risk factors, including gender and occupational exposure.
Parameters and measurements
The independent parameters examined comprised two categories, including individual factors (gender, occupation, and education) and biotic environmental factors (flood history, drainage conditions, and waste disposal conditions). The dependent parameter was leptospirosis status (case/control). Standardized operational definitions were established for all parameters to ensure measurement consistency. The occupational risk was dichotomized based on established exposure risk categorizations from previous epidemiological studies, with agricultural workers, sewage handlers, and animal handlers classified as high-risk occupations. Environmental parameters were assessed using validated criteria for infrastructure quality and hydrological risk, with drainage conditions evaluated based on flow characteristics, overflow frequency, and evidence of vector presence. Educational status was categorized according to national educational attainment standards to ensure contextual relevance and interpretability within the local socioeconomic framework.
Data collection procedures
Data collection proceeded through two complementary approaches to ensure a comprehensive assessment of parameters. Primary data acquisition involved the administration of structured questionnaires to all participants following thorough informed consent procedures. The questionnaire included sections on sociodemographic characteristics, occupational exposures, and environmental conditions, which were completed through direct participant interviews conducted by trained research personnel to minimize measurement bias and ensure standardized assessment.
Environmental condition assessment relied on structured questionnaire items evaluating flood history, drainage infrastructure quality, and waste disposal conditions using operationalized definitions to maximize consistency. The environmental evaluation approach was selected following systematic consideration of alternative methodologies, including objective environmental sampling. While direct environmental assessment (microbiological sampling of water sources and standardized infrastructure evaluation) would provide more precise exposure classification, resource constraints precluded the implementation of comprehensive environmental sampling protocols across the study catchment area. The limitations inherent in self-reported environmental data were explicitly addressed in the discussion section, with appropriate interpretive caution regarding exposure classification accuracy.
Secondary data collection involved accessing medical records to verify case status and clinical parameters, including laboratory confirmation data employing microscopic agglutination testing or molecular diagnostics. Additional contextual information was obtained from district health office surveillance records and published epidemiological reports to validate case identification and characterize the broader disease distribution pattern. Data triangulation processes were implemented to reconcile information from multiple sources and enhance the validity of classification decisions.
Statistical analysis
Data analysis proceeded sequentially through univariate and bivariate phases using established epidemiological analytical frameworks. Univariate analysis generated descriptive statistics to characterize frequency distributions for all study parameters, including categorical data presented through proportions and percentages to establish baseline characteristics of the study population. Bivariate analysis employed Chi-square tests to examine associations between independent parameters and leptospirosis status, with statistical significance defined at α=0.05. For parameters with expected cell counts below theoretical minimums, Fisher’s exact test was applied as an alternative procedure. Odds ratios with corresponding 95% confidence intervals were calculated using Mantel-Haenszel procedures to quantify the magnitude and directionality of associations. Risk factor interpretation followed established epidemiological criteria wherein OR>1 with 95% CI excluding 1 indicated a confirmed risk factor, OR>1 with 95% CI including one suggested a potential but unconfirmed risk factor, and OR<1 represented potential protective factors.
This conventional analytical approach provided a robust assessment of dichotomous risk associations but precluded more sophisticated spatial analysis that would enable the evaluation of geographical clustering, risk surface modeling, and integrated assessment of environmental-socioeconomic interactions. Resource constraints and methodological considerations regarding sample size adequacy for multivariate modeling influenced the selection of analytical methods, with priority placed on ensuring statistical validity within the limitations of available data. The methodological constraints inherent in this analytical framework are addressed in the discussion section, with specific recommendations for advanced geospatial modeling approaches in future investigations.
Findings
Sociodemographic characteristics
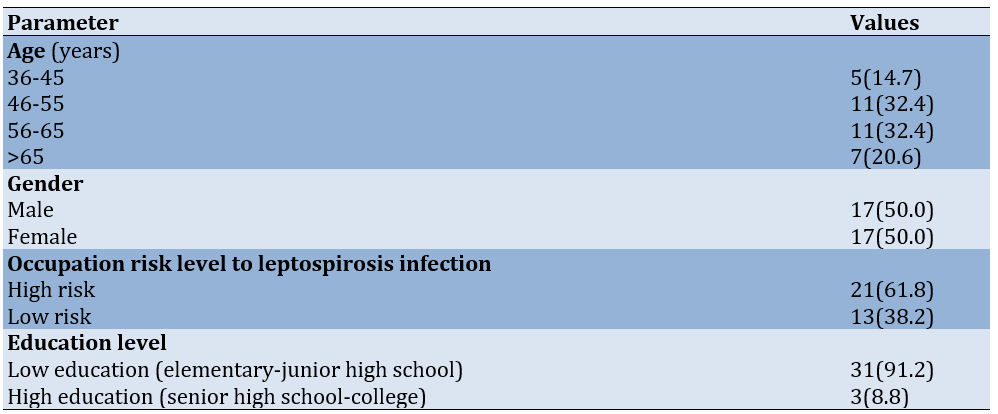
Sociodemographic analysis of the 34 study participants revealed that the age distribution was predominantly concentrated in the middle to older adult categories, with equal proportions (32.4%) in both the 46-55 and 56-65 age brackets (11 participants each). The remaining participants were distributed among the 36-45 age category (14.7%) and those above 65 years (20.6%). Gender distribution demonstrated perfect equilibrium, with 17 male and 17 female participants, each constituting 50.0% of the sample population. Occupational risk stratification identified 21 participants (61.8%) engaged in high-risk occupations, compared to 13 participants (38.2%) in occupations classified as low-risk. Educational attainment analysis revealed pronounced asymmetry, with 31 participants (91.2%) classified as having low educational status compared to only three participants (8.8%) with higher educational attainment (Table 1).
Table 1. Univariate analysis of the frequency of individual factors in the working area of Kasihan II Bantul public health center (n=34)
Environmental risk factors
The majority of participants (29, 85.3%) inhabited areas with good flood history (absence of significant flooding events), while only five participants (14.7%) resided in are:as char:acterized by poor flood history (recurring inundation episodes). Also, 19 participants (55.9%) had access to good drainage systems characterized by proper flow, the absence of overflow during precipitation, and limited rodent presence. Conversely, 15 participants (44.1%) were exposed to poor drainage conditions lacking one or more quality criteria. Waste disposal assessment identified suboptimal conditions among 20 participants (58.8%), contrasting with 14 participants (41.2%) who had access to adequate waste management infrastructure (Table 2).
Table 2. Univariate analysis of the frequency of biotic environmental factors in the working area of Kasihan II Bantul public health center (n=34)

Association between risk factors and leptospirosis
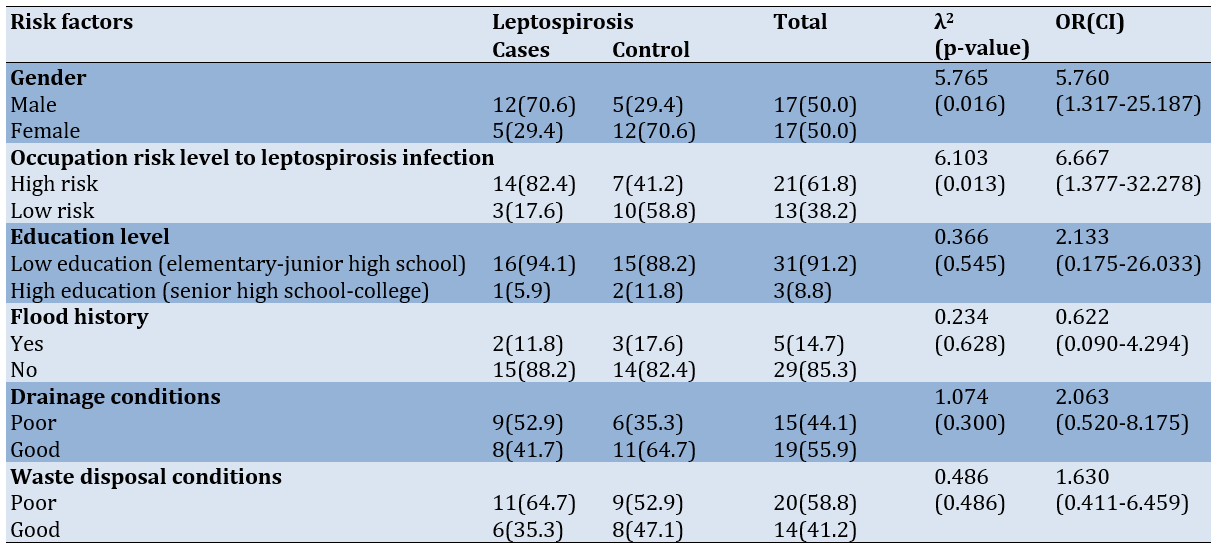
Bivariate analysis on the relationship between hypothesized risk factors and leptospirosis status revealed significant associations for selected parameters, while others demonstrated no statistically significant relationship. Gender demonstrated a significant association with leptospirosis (χ²=5.765, p=0.016), with males exhibiting substantially higher risk (OR=5.760, 95% CI: 1.317-25.187) compared to females. Males constituted 70.6% of the case group compared to 29.4% of controls, establishing gender as a significant risk determinant.
Occupational risk classification similarly demonstrated a significant association with leptospirosis incidence (χ²=6.103, p=0.013). High-risk occupations conferred substantially elevated disease probability (OR=6.667, 95% CI: 1.377-32.278) compared to low-risk occupational categories. This association was reflected in the disproportionate representation of high-risk occupations among cases (82.4%) compared to controls (41.2%).
Educational status did not demonstrate a statistically significant association with leptospirosis incidence (χ²=0.366, p=0.545), despite an elevated odds ratio (OR=2.133, 95% CI: 0.175-26.033) suggesting a potential relationship. The wide confidence interval encompassing the null value indicates substantial uncertainty regarding the true magnitude of this association. The environmental parameters examined, including flood history (χ²=0.234, p=0.628), drainage conditions (χ²=1.074, p=0.300), and waste disposal conditions (χ²=0.486, p=0.486), similarly failed to demonstrate statistically significant associations with leptospirosis incidence. The absence of significant findings for these environmental determinants contradicts established epidemiological patterns observed in other geographic contexts (Table 3).
Table 3. Association between frequency of leptospirosis risk factors and leptospirosis incidence in the working area of Kasihan II Bantul public health center
Discussion
This case-control study sought to examine the relationship between individual factors (gender, occupation, and education) and biotic environmental parameters (flood history, drainage conditions, and waste disposal conditions) with leptospirosis incidence in the working area of the Kasihan II Bantul Public Health Center during 2022-2023. There were significant associations between male gender and high-risk occupations with leptospirosis occurrence, establishing these as independent risk determinants in this endemic setting. Conversely, educational status and environmental factors, including flood history, drainage conditions, and waste disposal infrastructure, did not demonstrate statistically significant associations with disease transmission, though wide confidence intervals suggest potential relationships that warrant further investigation with larger sample populations.
Male gender and high-risk occupational exposure were significant determinants of leptospirosis. These findings align with established epidemiological patterns documented across diverse geographical contexts and provide localized evidence for targeted intervention development. The gender disparity observed in this study population reflects consistent patterns documented in global leptospirosis literature, where males consistently demonstrate heightened vulnerability across endemic regions [28, 29]. This gender-associated risk emerges from complex intersections between biological susceptibility, behavioral patterns, and socially constructed gender roles that influence exposure probability through multiple pathways.
The identified occupational risk profile demonstrated strong coherence with the established occupational epidemiology of leptospirosis. High-risk occupations, particularly agricultural work, construction labor, and fishing activities, constituted a substantial majority of cases compared to controls, reinforcing occupation-mediated exposure as a primary determinant of infection probability. This finding corroborates international evidence positioning agricultural workers, particularly rice farmers, among the highest-risk occupational categories [30-34]. The vulnerability of agricultural workers stems from sustained environmental exposure to potentially contaminated soil and water sources, creating multiple opportunities for pathogen transmission through cutaneous routes. Our findings regarding construction laborers (particularly “kuli bangunan”) align with emerging evidence recognizing urban service workers as an increasingly important risk group in developing regions, reflecting changing urbanization patterns and occupational distributions [35-37].
The intersection between gender and occupation creates compounded vulnerability profiles that warrant integrated analytical consideration. The gender-based division of labor documented in our study region parallels broader patterns reported in high-endemic areas, where occupations involving the highest exposure risk disproportionately employ male workers [31, 34, 37, 38]. This occupational stratification partially explains the observed gender disparity in leptospirosis distribution, though behavioral factors likely contribute additional risk dimensions. The limited adoption of personal protective equipment, particularly appropriate footwear during agricultural activities, emerges as a critical behavioral determinant consistent with patterns observed in seroprevalence studies of high-risk occupational groups [32]. Environmental exposure through recreational activities, including fishing and interaction with natural water bodies, represents an additional gender-modulated risk pathway extensively documented in previous research [33, 39, 40].
Moreover, there was no significant association between educational attainment and leptospirosis incidence. This finding corresponds with previous epidemiological research from Sri Lanka that has demonstrated the presence of substantial knowledge disparities regarding transmission mechanisms and prophylactic measures across educational strata, despite relatively uniform recognition of rodents as reservoir hosts [41]. Complementary evidence from Trinidad and Tobago further elucidates this complexity, documenting that geographical proximity to endemic foci functions as a stronger predictor of leptospirosis awareness than educational attainment metrics [42]. These findings collectively suggest that contextual determinants, including localized disease prevalence patterns, community-level health literacy initiatives, and regionalized risk communication strategies, may significantly modulate the translation of formal education into protective behaviors.
However, there were no significant associations between environmental factors, including flood history, drainage conditions, waste disposal infrastructure, and leptospirosis incidence in the Kasihan II Bantul catchment area. These results contrast with established literature documenting environmental determinants as critical factors in leptospirosis epidemiology across diverse geographical contexts [43-48]. The absence of significant associations may reflect several methodological considerations, including limited statistical power due to sample size constraints, potential exposure misclassification in retrospective assessments, and high environmental homogeneity within the study area. Additionally, the predominance of good flood history among participants restricted the analytical capacity to detect flood-associated risk patterns, despite substantial evidence from comparable investigations establishing flooding as a significant amplifier of transmission probability.
The analysis of drainage infrastructure and waste management revealed elevated risk estimates that failed to reach statistical significance. These environmental parameters possess established biological plausibility in leptospirosis transmission dynamics through their influence on vector ecology and the environmental persistence of pathogenic leptospires. The findings align with research from a previous study documenting similar non-significant associations between drainage conditions and leptospirosis [49]. The complex interplay between environmental parameters and disease transmission appears to be modulated by additional factors not captured in the analytical framework, including seasonal variation in precipitation patterns, vector population dynamics, and localized hydrological characteristics that influence environmental contamination profiles across the study region.
This study provides valuable insights into the epidemiological determinants of leptospirosis in the Kasihan II Bantul region, with particular emphasis on the significant associations between individual factors and disease occurrence. The identification of male gender and high-risk occupations as significant risk determinants offers critical evidence for targeted public health interventions in this endemic context. While environmental parameters demonstrated elevated risk estimates without reaching statistical significance, these findings contribute to the evolving discourse on leptospirosis transmission dynamics in Indonesia.
This investigation encountered several methodological constraints warranting critical consideration. First, the modest sample size (n=34) limited statistical power for detecting moderate-effect associations, potentially obscuring significant relationships, particularly for environmental parameters. Second, the quasi-experimental design without randomization introduces potential selection bias, despite efforts to ensure between-group comparability. Third, reliance on self-reported environmental conditions without objective microbiological sampling or standardized infrastructure assessments created potential exposure misclassification. Fourth, demographic homogeneity (91.2% with low education; 85.3% reporting good flood history) restricted the analytical capacity to detect associations within these domains. Fifth, the cross-sectional temporal structure precluded the evaluation of seasonal variability, lag effects, and long-term impact trajectories. Sixth, geographical restriction to a single catchment area constrained the representation of environmental heterogeneity and limited generalizability beyond Kasihan II Bantul. Finally, conventional bivariate analytical approaches without advanced geospatial modeling techniques prevented a comprehensive assessment of spatial heterogeneity and environmental-socioeconomic interactions that may potentially modulate transmission dynamics. These limitations necessitate cautious interpretation, particularly regarding environmental parameters, where statistical power constraints and measurement limitations may have obscured genuine associations. The significant relationships identified between the male gender and high-risk occupations with leptospirosis demonstrate robust statistical parameters despite methodological constraints, enhancing confidence in these specific findings. However, the absence of significant associations for environmental factors should be interpreted as inconclusive rather than definitively negative, given the intersecting limitations affecting these parameters.
The findings have substantial implications for public health practice and research methodology. Evidence-based prevention strategies should prioritize occupation-specific interventions targeting agricultural workers, construction laborers, and fishing communities through multifaceted approaches, including enhanced access to personal protective equipment, context-appropriate educational modules, and workplace environmental modifications. Targeted community engagement and education programs should be implemented with particular emphasis on high-risk occupational groups, utilizing established behavior change frameworks to develop tailored interventions that address risk perception, protective behaviors, and environmental management. Comprehensive policy frameworks should be developed across multiple domains, including occupational safety regulations requiring appropriate protective equipment for high-risk workers; community-level environmental modifications addressing drainage infrastructure and waste management; and enhanced surveillance systems with occupation-specific monitoring components.
Future research should address identified methodological limitations through multicenter recruitment strategies across diverse endemic regions, including Bantul, Sewon, and Pundong sub-districts, to enhance sample size and demographic heterogeneity, prospective cohort designs with longitudinal assessments incorporating strategic temporal sampling intervals aligned with established seasonal transmission patterns, objective environmental assessment protocols, including microbiological sampling and standardized infrastructure evaluation, advanced analytical frameworks incorporating geospatial modeling techniques to integrate environmental and socioeconomic determinants, and mixed-methods evaluations of targeted interventions across multiple outcome domains. These methodological refinements would substantially enhance understanding of complex leptospirosis transmission dynamics while facilitating the development of increasingly precise prevention strategies tailored to local epidemiological contexts.
Conclusion
In the Kasihan II Bantul catchment area, the significant determinants of leptospirosis transmission are male gender and high-risk occupational exposure, while educational status and environmental factors are non-significant determinants.
Acknowledgments: The authors express their gratitude to the study participants for their involvement and generous contributions. They also extend their special thanks to Puskesmas Kasihan Bantul II for their invaluable data support and contributions to the research.
Ethical Permissions: Ethical permission for this research was obtained on 14 May 2024, with protocol number 2.14/KEPK/SSG/V/2024 from the Ethical Committee of Health Research of STIKES Surya Global Yogyakarta. Before collecting data, the researchers obtained informed consent from all study participants. Throughout this research, ethical principles, such as participant confidentiality and autonomy were strictly upheld. Each participant was granted the freedom to withdraw from the study at any time if they felt uncomfortable, and there were no consequences for doing so. The investigation adhered to the principles of the Declaration of Helsinki and local regulatory requirements throughout all research phases, with particular attention to respectful community engagement practices and considerations of potential vulnerability.
Conflicts of Interests: The authors reported no conflicts of interests.
Authors' Contribution: Istanti Y (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (60%); Adnani H (Second Author), Introduction Writer/Methodologist/Discussion Writer/Statistical Analyst (40%)
Funding/Support: This study does not receive any external funding.
Leptospirosis is a significant zoonotic disease with a global distribution, particularly affecting tropical, subtropical, and temperate regions. The disease is prevalent in areas such as the Caribbean, Latin America, the Indian subcontinent, Southeast Asia, Oceania, and parts of Eastern Europe [1]. Globalization and international travel have also increased exposure to leptospirosis in developed countries, expanding its epidemiological footprint beyond traditional endemic zones [1]. The Asia-Pacific region is notably affected, with outbreaks often linked to poor sanitation, overcrowding, and climatic conditions that favor bacterial persistence and transmission [2]. These demographic and environmental determinants create complex transmission dynamics that challenge public health control efforts across diverse geographical settings.
Leptospirosis is often underdiagnosed, leading to an underestimation of its global burden. The World Health Organization (WHO) has established the Leptospirosis Burden Epidemiology Reference Group (LERG) to estimate the global burden using disability-adjusted life years (DALYs) as a standardized metric [3]. Current epidemiological data indicate an estimated annual incidence of approximately half a million severe human cases globally; however, this figure likely represents substantial underreporting due to diagnostic limitations and surveillance inadequacies [4]. Mortality rates demonstrate significant variation, with higher rates documented in older patients and those presenting with severe clinical manifestations such as jaundice and renal failure [5]. The burden of disease extends beyond acute mortality, with persistent post-infection sequelae, including fatigue and myalgia, contributing to long-term morbidity and reduced quality of life [6].
Leptospirosis is caused by spirochetes of the genus Leptospira, with pathophysiological mechanisms that produce a remarkably diverse clinical spectrum. Disease presentations range from mild febrile illness to severe complications, including hepatorenal dysfunction, myocarditis, pulmonary hemorrhage, and meningitis [7]. The pathophysiology involves bacterial entry through mucous membranes or skin abrasions, often due to exposure to contaminated water sources, followed by hematogenous dissemination that affects multiple organ systems [7]. This complex pathophysiological profile creates diagnostic challenges and contributes to the underestimation of the disease burden, particularly in regions with limited laboratory capacity. The interplay between bacterial virulence factors, host immune responses, and environmental exposures creates a multifaceted disease entity that requires integrated surveillance and management approaches across the healthcare continuum.
Previous systematic reviews indicate that leptospirosis carries substantial morbidity and mortality metrics; however, it remains underrepresented in global health prioritization frameworks, resulting in fragmented surveillance systems and limited intervention development [8, 9]. The disease exhibits pronounced socioeconomic stratification patterns, with the highest incidence documented among marginalized communities where inadequate sanitation infrastructure, occupational exposures, and limited healthcare access create synergistic risk profiles [10-12]. Epidemiological analyses demonstrate that agricultural workers, fishermen, sewage workers, and inhabitants of informal settlements face exponentially higher exposure probabilities, reflecting the intersection between environmental determinants and structural inequalities in disease distribution [10, 12].
The persistent neglect and substantial underreporting of leptospirosis present formidable challenges to accurate burden quantification, with current surveillance systems capturing only an estimated 5-15% of actual cases across endemic regions [8, 13, 14]. This surveillance inadequacy stems from multiple intersecting factors, including the non-specific clinical presentation that frequently mimics other febrile illnesses, leading to misdiagnosis and classification errors within healthcare systems [15, 16]. Diagnostic complexity represents a fundamental barrier to effective surveillance, as the disease manifests along a broad clinical spectrum ranging from mild influenza-like illness to severe manifestations, encompassing Weil’s disease with its characteristic triad of jaundice, renal failure, and hemorrhagic phenomena [9, 11, 12, 16].
Leptospirosis determinants comprise interrelated environmental and socioeconomic factors that create complex risk landscapes with significant geographical and temporal heterogeneity. Environmental parameters, including temperature, precipitation, flooding events, and humidity levels, demonstrate consistent associations with disease incidence, with multivariate analyses identifying annual mean temperature and rainfall metrics as significant predictors of transmission intensity [17-19]. Hydrological factors, particularly flood exposure, function as critical risk amplifiers (incidence rate ratios: 2.3-8.7) [20, 21], while geographical characteristics, including land cover typologies, altitudinal gradients, and proximity to water bodies, further modulate spatial distribution patterns [18, 22]. These environmental determinants interact synergistically with socioeconomic factors, with quantitative evidence establishing significant associations between the leptospirosis burden and urbanization patterns, especially in informal settlements characterized by high population density and inadequate sanitation infrastructure [20, 23].
Housing quality metrics demonstrate strong correlations with infection risk (odds ratios: 2.21-5.15 for substandard dwellings) [23], while economic vulnerability indicators, including poverty rates and educational attainment, consistently predict disease distribution through pathways involving reduced access to protective infrastructure, occupational exposures, and limited healthcare utilization [23-25]. Advanced geospatial modeling approaches that incorporate these determinants enable high-resolution risk mapping and targeted intervention deployment, with vulnerability indices constructed from composite socioeconomic indicators providing evidence-based frameworks for public health resource allocation in endemic regions [18, 19, 22, 23].
Leptospirosis cases in Indonesia have shown an increasing trend annually. In 2020, there were 1,170 cases with 106 deaths (case fatality rate (CFR) of 9.06%, significantly higher than COVID-19 mortality), while in 2021, there were 736 cases with 84 deaths (CFR 11.41%). In 2022, based on reports from 11 provinces, there were 1,408 leptospirosis cases with 139 deaths (CFR 9.87%) [26]. Leptospirosis is believed to have the most widespread distribution globally and is known in some countries as “Rat Urine Fever.” High-risk populations include those whose occupations, living environments, or lifestyles expose them to the pathogen. High transmission risk occurs among farmers, plantation workers, pet shop employees, livestock handlers, sewer workers, slaughterhouse workers, meat processors, and military personnel [27].
According to the Yogyakarta Special Region Health Office, hundreds of leptospirosis cases are distributed across all districts and cities in the region. The highest number of cases was reported in Bantul Regency, reaching 110 cases. Yogyakarta City recorded 19 cases, Kulonprogo Regency recorded 36 cases, Gunungkidul Regency reported 56 cases, and Sleman recorded 42 cases [27]. Based on data from the Bantul District Health Office, leptospirosis cases in 2022 were 141 across 17 sub-districts, with the highest distribution in Kasihan Sub-district (15 cases) and Bantul Sub-district (13 cases), followed by Sandakan and Jetis Sub-districts (12 cases each), and Imogiri, Pundong, and Sewon Sub-districts (11 cases each). Pandak Sub-district reported 10 cases, Bambanglipuro Sub-district had 9 cases, Pleret Sub-district had 7 cases, and Piyungan and Sedayu Sub-districts each reported 6 cases. Banguntapan Sub-district had 5 cases, the Kretek, Pajangan, and Sanden Sub-districts each recorded 4 cases, and Dlingo Sub-district had 2 cases [27].
According to data from the Bantul District Health Office, leptospirosis cases in 2023 totaled 155 across 17 sub-districts, with the highest distribution in Bantul Sub-district (24 cases), followed by Sewon Sub-district (18 cases), Kasihan Sub-district (17 cases), and Pundong Sub-district (16 cases). Bambanglipuro and Pandak Sub-districts each reported 12 cases, while Sedayu Sub-district had 9 cases. Imogiri, Jetis, and Sanden Sub-districts each recorded 7 cases, and Banguntapan and Kretek Sub-districts each had 5 cases. Pleret and Srandakan Sub-districts each reported 2 cases [27].
Despite extensive epidemiological documentation of the global leptospirosis burden, significant knowledge deficits persist regarding localized transmission determinants in endemic Indonesian regions, particularly in the Yogyakarta Special Region, where case fatality rates consistently exceed 9%, markedly higher than COVID-19 mortality metrics. While surveillance data demonstrate distinct spatial clustering, with the Kasihan sub-district maintaining a high incidence, analytical investigations quantifying the relative contributions of individual versus environmental risk factors within this high-transmission zone remain conspicuously absent from the current evidence base.
This study, therefore, aimed to examine the relationship between individual factors (gender, occupation, education) and biotic environmental parameters (flood history, drainage conditions, waste disposal systems) with leptospirosis incidence in the Kasihan II Bantul Public Health Center catchment area during 2022-2023, employing a case-control analytical framework to identify high-priority modifiable determinants for targeted public health interventions aligned with local epidemiological patterns and resource constraints.
Instrument and Methods
Study design
This observational analytical study employed a retrospective case-control approach to investigate the relationship between individual and environmental factors and leptospirosis incidence. This methodological framework was selected to enable a quantitative assessment of risk factor associations by comparing exposure histories between individuals with confirmed leptospirosis diagnoses and matched controls without the disease. The retrospective orientation facilitated the examination of temporal relationships between potential risk factors and disease occurrence while maximizing efficiency within the constraints of available resources and the epidemiological context.
The case-control methodology was chosen after systematic consideration of alternative designs, including prospective cohort approaches. The retrospective design offered practical advantages in the study context, including feasibility for investigating relatively uncommon outcomes within resource and temporal constraints, capacity for simultaneous evaluation of multiple exposure parameters, and established precedent in the leptospirosis epidemiological literature, facilitating cross-study comparisons. However, this approach introduced methodological limitations, including potential recall bias in exposure assessment and the inability to establish precise temporal sequences between environmental exposures and disease occurrence. These limitations were explicitly addressed in the discussion section, with appropriate interpretive caution regarding causal inference.
Population and sampling
The target population comprised all leptospirosis patients residing within the catchment area of the Kasihan II Bantul Public Health Center. A total sampling technique was implemented to recruit all eligible cases diagnosed during the 2022-2023 study period, yielding 17 confirmed leptospirosis patients. Controls were selected using a 1:1 matching ratio, with 17 individuals without leptospirosis recruited from the same geographic area, resulting in a total sample of 34 participants.
Sample size considerations included a retrospective power analysis demonstrating adequate statistical capacity (1-β=0.80, α=0.05) to detect large effect sizes (OR≥5.0) but limited power for moderate (OR=2.0-3.0) or small effect associations. This statistical parameter was deemed acceptable for primary risk factors with established high-magnitude associations based on previous epidemiological investigations, though it may be insufficient for detecting environmental determinants with more modest effect sizes. The sampling approach was designed to maximize analytical validity within the constraints of case availability in this endemic region, with case identification protocols that included a comprehensive surveillance system review to ensure the inclusion of all laboratory-confirmed infections during the study period.
Inclusion criteria for cases encompassed a laboratory-confirmed leptospirosis diagnosis through microscopic agglutination testing or PCR methodologies and current residence within the study area. Controls were selected from the same communities to ensure comparable exposure opportunities to environmental risk factors. Control recruitment employed frequency matching to maintain demographic comparability in age distribution while allowing for analytical assessment of other hypothesized risk factors, including gender and occupational exposure.
Parameters and measurements
The independent parameters examined comprised two categories, including individual factors (gender, occupation, and education) and biotic environmental factors (flood history, drainage conditions, and waste disposal conditions). The dependent parameter was leptospirosis status (case/control). Standardized operational definitions were established for all parameters to ensure measurement consistency. The occupational risk was dichotomized based on established exposure risk categorizations from previous epidemiological studies, with agricultural workers, sewage handlers, and animal handlers classified as high-risk occupations. Environmental parameters were assessed using validated criteria for infrastructure quality and hydrological risk, with drainage conditions evaluated based on flow characteristics, overflow frequency, and evidence of vector presence. Educational status was categorized according to national educational attainment standards to ensure contextual relevance and interpretability within the local socioeconomic framework.
Data collection procedures
Data collection proceeded through two complementary approaches to ensure a comprehensive assessment of parameters. Primary data acquisition involved the administration of structured questionnaires to all participants following thorough informed consent procedures. The questionnaire included sections on sociodemographic characteristics, occupational exposures, and environmental conditions, which were completed through direct participant interviews conducted by trained research personnel to minimize measurement bias and ensure standardized assessment.
Environmental condition assessment relied on structured questionnaire items evaluating flood history, drainage infrastructure quality, and waste disposal conditions using operationalized definitions to maximize consistency. The environmental evaluation approach was selected following systematic consideration of alternative methodologies, including objective environmental sampling. While direct environmental assessment (microbiological sampling of water sources and standardized infrastructure evaluation) would provide more precise exposure classification, resource constraints precluded the implementation of comprehensive environmental sampling protocols across the study catchment area. The limitations inherent in self-reported environmental data were explicitly addressed in the discussion section, with appropriate interpretive caution regarding exposure classification accuracy.
Secondary data collection involved accessing medical records to verify case status and clinical parameters, including laboratory confirmation data employing microscopic agglutination testing or molecular diagnostics. Additional contextual information was obtained from district health office surveillance records and published epidemiological reports to validate case identification and characterize the broader disease distribution pattern. Data triangulation processes were implemented to reconcile information from multiple sources and enhance the validity of classification decisions.
Statistical analysis
Data analysis proceeded sequentially through univariate and bivariate phases using established epidemiological analytical frameworks. Univariate analysis generated descriptive statistics to characterize frequency distributions for all study parameters, including categorical data presented through proportions and percentages to establish baseline characteristics of the study population. Bivariate analysis employed Chi-square tests to examine associations between independent parameters and leptospirosis status, with statistical significance defined at α=0.05. For parameters with expected cell counts below theoretical minimums, Fisher’s exact test was applied as an alternative procedure. Odds ratios with corresponding 95% confidence intervals were calculated using Mantel-Haenszel procedures to quantify the magnitude and directionality of associations. Risk factor interpretation followed established epidemiological criteria wherein OR>1 with 95% CI excluding 1 indicated a confirmed risk factor, OR>1 with 95% CI including one suggested a potential but unconfirmed risk factor, and OR<1 represented potential protective factors.
This conventional analytical approach provided a robust assessment of dichotomous risk associations but precluded more sophisticated spatial analysis that would enable the evaluation of geographical clustering, risk surface modeling, and integrated assessment of environmental-socioeconomic interactions. Resource constraints and methodological considerations regarding sample size adequacy for multivariate modeling influenced the selection of analytical methods, with priority placed on ensuring statistical validity within the limitations of available data. The methodological constraints inherent in this analytical framework are addressed in the discussion section, with specific recommendations for advanced geospatial modeling approaches in future investigations.
Findings
Sociodemographic characteristics
Sociodemographic analysis of the 34 study participants revealed that the age distribution was predominantly concentrated in the middle to older adult categories, with equal proportions (32.4%) in both the 46-55 and 56-65 age brackets (11 participants each). The remaining participants were distributed among the 36-45 age category (14.7%) and those above 65 years (20.6%). Gender distribution demonstrated perfect equilibrium, with 17 male and 17 female participants, each constituting 50.0% of the sample population. Occupational risk stratification identified 21 participants (61.8%) engaged in high-risk occupations, compared to 13 participants (38.2%) in occupations classified as low-risk. Educational attainment analysis revealed pronounced asymmetry, with 31 participants (91.2%) classified as having low educational status compared to only three participants (8.8%) with higher educational attainment (Table 1).
Table 1. Univariate analysis of the frequency of individual factors in the working area of Kasihan II Bantul public health center (n=34)

Environmental risk factors
The majority of participants (29, 85.3%) inhabited areas with good flood history (absence of significant flooding events), while only five participants (14.7%) resided in are:as char:acterized by poor flood history (recurring inundation episodes). Also, 19 participants (55.9%) had access to good drainage systems characterized by proper flow, the absence of overflow during precipitation, and limited rodent presence. Conversely, 15 participants (44.1%) were exposed to poor drainage conditions lacking one or more quality criteria. Waste disposal assessment identified suboptimal conditions among 20 participants (58.8%), contrasting with 14 participants (41.2%) who had access to adequate waste management infrastructure (Table 2).
Table 2. Univariate analysis of the frequency of biotic environmental factors in the working area of Kasihan II Bantul public health center (n=34)

Association between risk factors and leptospirosis
Bivariate analysis on the relationship between hypothesized risk factors and leptospirosis status revealed significant associations for selected parameters, while others demonstrated no statistically significant relationship. Gender demonstrated a significant association with leptospirosis (χ²=5.765, p=0.016), with males exhibiting substantially higher risk (OR=5.760, 95% CI: 1.317-25.187) compared to females. Males constituted 70.6% of the case group compared to 29.4% of controls, establishing gender as a significant risk determinant.
Occupational risk classification similarly demonstrated a significant association with leptospirosis incidence (χ²=6.103, p=0.013). High-risk occupations conferred substantially elevated disease probability (OR=6.667, 95% CI: 1.377-32.278) compared to low-risk occupational categories. This association was reflected in the disproportionate representation of high-risk occupations among cases (82.4%) compared to controls (41.2%).
Educational status did not demonstrate a statistically significant association with leptospirosis incidence (χ²=0.366, p=0.545), despite an elevated odds ratio (OR=2.133, 95% CI: 0.175-26.033) suggesting a potential relationship. The wide confidence interval encompassing the null value indicates substantial uncertainty regarding the true magnitude of this association. The environmental parameters examined, including flood history (χ²=0.234, p=0.628), drainage conditions (χ²=1.074, p=0.300), and waste disposal conditions (χ²=0.486, p=0.486), similarly failed to demonstrate statistically significant associations with leptospirosis incidence. The absence of significant findings for these environmental determinants contradicts established epidemiological patterns observed in other geographic contexts (Table 3).
Table 3. Association between frequency of leptospirosis risk factors and leptospirosis incidence in the working area of Kasihan II Bantul public health center

Discussion
This case-control study sought to examine the relationship between individual factors (gender, occupation, and education) and biotic environmental parameters (flood history, drainage conditions, and waste disposal conditions) with leptospirosis incidence in the working area of the Kasihan II Bantul Public Health Center during 2022-2023. There were significant associations between male gender and high-risk occupations with leptospirosis occurrence, establishing these as independent risk determinants in this endemic setting. Conversely, educational status and environmental factors, including flood history, drainage conditions, and waste disposal infrastructure, did not demonstrate statistically significant associations with disease transmission, though wide confidence intervals suggest potential relationships that warrant further investigation with larger sample populations.
Male gender and high-risk occupational exposure were significant determinants of leptospirosis. These findings align with established epidemiological patterns documented across diverse geographical contexts and provide localized evidence for targeted intervention development. The gender disparity observed in this study population reflects consistent patterns documented in global leptospirosis literature, where males consistently demonstrate heightened vulnerability across endemic regions [28, 29]. This gender-associated risk emerges from complex intersections between biological susceptibility, behavioral patterns, and socially constructed gender roles that influence exposure probability through multiple pathways.
The identified occupational risk profile demonstrated strong coherence with the established occupational epidemiology of leptospirosis. High-risk occupations, particularly agricultural work, construction labor, and fishing activities, constituted a substantial majority of cases compared to controls, reinforcing occupation-mediated exposure as a primary determinant of infection probability. This finding corroborates international evidence positioning agricultural workers, particularly rice farmers, among the highest-risk occupational categories [30-34]. The vulnerability of agricultural workers stems from sustained environmental exposure to potentially contaminated soil and water sources, creating multiple opportunities for pathogen transmission through cutaneous routes. Our findings regarding construction laborers (particularly “kuli bangunan”) align with emerging evidence recognizing urban service workers as an increasingly important risk group in developing regions, reflecting changing urbanization patterns and occupational distributions [35-37].
The intersection between gender and occupation creates compounded vulnerability profiles that warrant integrated analytical consideration. The gender-based division of labor documented in our study region parallels broader patterns reported in high-endemic areas, where occupations involving the highest exposure risk disproportionately employ male workers [31, 34, 37, 38]. This occupational stratification partially explains the observed gender disparity in leptospirosis distribution, though behavioral factors likely contribute additional risk dimensions. The limited adoption of personal protective equipment, particularly appropriate footwear during agricultural activities, emerges as a critical behavioral determinant consistent with patterns observed in seroprevalence studies of high-risk occupational groups [32]. Environmental exposure through recreational activities, including fishing and interaction with natural water bodies, represents an additional gender-modulated risk pathway extensively documented in previous research [33, 39, 40].
Moreover, there was no significant association between educational attainment and leptospirosis incidence. This finding corresponds with previous epidemiological research from Sri Lanka that has demonstrated the presence of substantial knowledge disparities regarding transmission mechanisms and prophylactic measures across educational strata, despite relatively uniform recognition of rodents as reservoir hosts [41]. Complementary evidence from Trinidad and Tobago further elucidates this complexity, documenting that geographical proximity to endemic foci functions as a stronger predictor of leptospirosis awareness than educational attainment metrics [42]. These findings collectively suggest that contextual determinants, including localized disease prevalence patterns, community-level health literacy initiatives, and regionalized risk communication strategies, may significantly modulate the translation of formal education into protective behaviors.
However, there were no significant associations between environmental factors, including flood history, drainage conditions, waste disposal infrastructure, and leptospirosis incidence in the Kasihan II Bantul catchment area. These results contrast with established literature documenting environmental determinants as critical factors in leptospirosis epidemiology across diverse geographical contexts [43-48]. The absence of significant associations may reflect several methodological considerations, including limited statistical power due to sample size constraints, potential exposure misclassification in retrospective assessments, and high environmental homogeneity within the study area. Additionally, the predominance of good flood history among participants restricted the analytical capacity to detect flood-associated risk patterns, despite substantial evidence from comparable investigations establishing flooding as a significant amplifier of transmission probability.
The analysis of drainage infrastructure and waste management revealed elevated risk estimates that failed to reach statistical significance. These environmental parameters possess established biological plausibility in leptospirosis transmission dynamics through their influence on vector ecology and the environmental persistence of pathogenic leptospires. The findings align with research from a previous study documenting similar non-significant associations between drainage conditions and leptospirosis [49]. The complex interplay between environmental parameters and disease transmission appears to be modulated by additional factors not captured in the analytical framework, including seasonal variation in precipitation patterns, vector population dynamics, and localized hydrological characteristics that influence environmental contamination profiles across the study region.
This study provides valuable insights into the epidemiological determinants of leptospirosis in the Kasihan II Bantul region, with particular emphasis on the significant associations between individual factors and disease occurrence. The identification of male gender and high-risk occupations as significant risk determinants offers critical evidence for targeted public health interventions in this endemic context. While environmental parameters demonstrated elevated risk estimates without reaching statistical significance, these findings contribute to the evolving discourse on leptospirosis transmission dynamics in Indonesia.
This investigation encountered several methodological constraints warranting critical consideration. First, the modest sample size (n=34) limited statistical power for detecting moderate-effect associations, potentially obscuring significant relationships, particularly for environmental parameters. Second, the quasi-experimental design without randomization introduces potential selection bias, despite efforts to ensure between-group comparability. Third, reliance on self-reported environmental conditions without objective microbiological sampling or standardized infrastructure assessments created potential exposure misclassification. Fourth, demographic homogeneity (91.2% with low education; 85.3% reporting good flood history) restricted the analytical capacity to detect associations within these domains. Fifth, the cross-sectional temporal structure precluded the evaluation of seasonal variability, lag effects, and long-term impact trajectories. Sixth, geographical restriction to a single catchment area constrained the representation of environmental heterogeneity and limited generalizability beyond Kasihan II Bantul. Finally, conventional bivariate analytical approaches without advanced geospatial modeling techniques prevented a comprehensive assessment of spatial heterogeneity and environmental-socioeconomic interactions that may potentially modulate transmission dynamics. These limitations necessitate cautious interpretation, particularly regarding environmental parameters, where statistical power constraints and measurement limitations may have obscured genuine associations. The significant relationships identified between the male gender and high-risk occupations with leptospirosis demonstrate robust statistical parameters despite methodological constraints, enhancing confidence in these specific findings. However, the absence of significant associations for environmental factors should be interpreted as inconclusive rather than definitively negative, given the intersecting limitations affecting these parameters.
The findings have substantial implications for public health practice and research methodology. Evidence-based prevention strategies should prioritize occupation-specific interventions targeting agricultural workers, construction laborers, and fishing communities through multifaceted approaches, including enhanced access to personal protective equipment, context-appropriate educational modules, and workplace environmental modifications. Targeted community engagement and education programs should be implemented with particular emphasis on high-risk occupational groups, utilizing established behavior change frameworks to develop tailored interventions that address risk perception, protective behaviors, and environmental management. Comprehensive policy frameworks should be developed across multiple domains, including occupational safety regulations requiring appropriate protective equipment for high-risk workers; community-level environmental modifications addressing drainage infrastructure and waste management; and enhanced surveillance systems with occupation-specific monitoring components.
Future research should address identified methodological limitations through multicenter recruitment strategies across diverse endemic regions, including Bantul, Sewon, and Pundong sub-districts, to enhance sample size and demographic heterogeneity, prospective cohort designs with longitudinal assessments incorporating strategic temporal sampling intervals aligned with established seasonal transmission patterns, objective environmental assessment protocols, including microbiological sampling and standardized infrastructure evaluation, advanced analytical frameworks incorporating geospatial modeling techniques to integrate environmental and socioeconomic determinants, and mixed-methods evaluations of targeted interventions across multiple outcome domains. These methodological refinements would substantially enhance understanding of complex leptospirosis transmission dynamics while facilitating the development of increasingly precise prevention strategies tailored to local epidemiological contexts.
Conclusion
In the Kasihan II Bantul catchment area, the significant determinants of leptospirosis transmission are male gender and high-risk occupational exposure, while educational status and environmental factors are non-significant determinants.
Acknowledgments: The authors express their gratitude to the study participants for their involvement and generous contributions. They also extend their special thanks to Puskesmas Kasihan Bantul II for their invaluable data support and contributions to the research.
Ethical Permissions: Ethical permission for this research was obtained on 14 May 2024, with protocol number 2.14/KEPK/SSG/V/2024 from the Ethical Committee of Health Research of STIKES Surya Global Yogyakarta. Before collecting data, the researchers obtained informed consent from all study participants. Throughout this research, ethical principles, such as participant confidentiality and autonomy were strictly upheld. Each participant was granted the freedom to withdraw from the study at any time if they felt uncomfortable, and there were no consequences for doing so. The investigation adhered to the principles of the Declaration of Helsinki and local regulatory requirements throughout all research phases, with particular attention to respectful community engagement practices and considerations of potential vulnerability.
Conflicts of Interests: The authors reported no conflicts of interests.
Authors' Contribution: Istanti Y (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (60%); Adnani H (Second Author), Introduction Writer/Methodologist/Discussion Writer/Statistical Analyst (40%)
Funding/Support: This study does not receive any external funding.
Article Type: Original Research |
Subject:
Social Determinants of Health
Received: 2025/02/10 | Accepted: 2025/03/12 | Published: 2025/04/28
Received: 2025/02/10 | Accepted: 2025/03/12 | Published: 2025/04/28
References
1. Pappas G, Papadimitriou P, Siozopoulou V, Christou L, Akritidis N. The globalization of leptospirosis: Worldwide incidence trends. Int J Infect Dis. 2008;12(4):351-7. [Link] [DOI:10.1016/j.ijid.2007.09.011]
2. Victoriano AFB, Smythe LD, Gloriani-Barzaga N, Cavinta LL, Kasai T, Limpakarnjanarat K, et al. Leptospirosis in the Asia Pacific region. BMC Infect Dis. 2009;9:147. [Link] [DOI:10.1186/1471-2334-9-147]
3. Abela-Ridder B, Sikkema R, Hartskeerl RA. Estimating the burden of human leptospirosis. Int J Antimicrob Agents. 2010;36(Suppl 1):S5-7. [Link] [DOI:10.1016/j.ijantimicag.2010.06.012]
4. Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: Dynamics of infection in the changing world. Clin Microbiol Infect. 2011;17(4):494-501. [Link] [DOI:10.1111/j.1469-0691.2011.03474.x]
5. Taylor AJ, Paris DH, Newton PN. A systematic review of the mortality from untreated leptospirosis. PLoS Negl Trop Dis. 2015;9(6). [Link] [DOI:10.1371/journal.pntd.0003866]
6. Goris MGA, Kikken V, Straetemans M, Alba S, Goeijenbier M, Van Gorp ECM, et al. Towards the burden of human leptospirosis: Duration of acute illness and occurrence of post-leptospirosis symptoms of patients in the Netherlands. PLoS One. 2013;8(10):e76549. [Link] [DOI:10.1371/journal.pone.0076549]
7. Rajput D, Gupta A, Verma SS, Barabari GS, Wani AA, Kumar N. Jaundice and thrombocytopenia in an acute abdomen with concurrent appendicitis and spontaneous rectal perforation: An unusual presentation of human leptospirosis. Trop Doct. 2021;51(3):427-31. [Link] [DOI:10.1177/0049475520981298]
8. Bradley EA, Lockaby G. Leptospirosis and the environment: A review and future directions. Pathogens. 2023;12(9):1167. [Link] [DOI:10.3390/pathogens12091167]
9. Chancharoenthana W, Leelahavanichkul A, Schultz MJ, Dondorp AM. Going micro in leptospirosis kidney disease. Cells. 2022;11(4):698. [Link] [DOI:10.3390/cells11040698]
10. Bouscaren N, Benoit De Coignac C, Lastère S, Musso D, Teissier Y, Formont J, et al. Leptospirosis in French Polynesia: 11 years of surveillance data, 2007-2017. New Microbes New Infect. 2019;29:100518. [Link] [DOI:10.1016/j.nmni.2019.100518]
11. Miguel PSB, De Carvalho Meireles MA, Feitosa RB, Da Motta OJR, De Oliveira Pereira S, Ribeiro AN, et al. Leptospirosis, a clinical update regarding a neglected infectious disease. J Trop Pathol. 2020;49(4):229-42. [Link] [DOI:10.5216/rpt.v49i4.67332]
12. Karpagam KB, Ganesh B. Leptospirosis: A neglected tropical zoonotic infection of public health importance-an updated review. Eur J Clin Microbiol Infect Dis. 2020;39(5):835-46. [Link] [DOI:10.1007/s10096-019-03797-4]
13. Cassadou S, Rosine J, Flamand C, Escher M, Ledrans M, Bourhy P, et al. Underestimation of leptospirosis incidence in the French west Indies. PLoS Negl Trop Dis. 2016;10(4). [Link] [DOI:10.1371/journal.pntd.0004668]
14. Arboleda M, Mejía-Torres M, Posada M, Restrepo N, Ríos-Tapias P, Rivera-Pedroza LA, et al. Molecular diagnosis as an alternative for public health surveillance of leptospirosis in Colombia. Microorganisms. 2023;11(11):2759. [Link] [DOI:10.3390/microorganisms11112759]
15. Levett PN. Leptospirosis: A forgotten zoonosis?. Clin Appl Immunol Rev. 2004;4(6):435-48. [Link] [DOI:10.1016/j.cair.2004.08.001]
16. Samrot AV, Sean TC, Bhavya KS, Sahithya CS, Chandrasekaran S, Palanisamy R, et al. Leptospiral infection, pathogenesis and its diagnosis-a review. Pathogens. 2021;10(2):145. [Link] [DOI:10.3390/pathogens10020145]
17. Kira R, Bilung LM, Ngui R, Apun K, Su'ut L. Spatially varying correlation between environmental conditions and human leptospirosis in Sarawak, Malaysia. Trop Biomed. 2021;38(2):31-9. [Link] [DOI:10.47665/tb.38.2.034]
18. Zakharova OI, Korennoy FI, Toropova NN, Burova OA, Blokhin AA. Environmental risk of leptospirosis in animals: The case of the Republic of Sakha (Yakutia), Russian Federation. Pathogens. 2020;9(6):504. [Link] [DOI:10.3390/pathogens9060504]
19. Zhao J, Liao J, Huang X, Zhao J, Wang Y, Ren J, et al. Mapping risk of leptospirosis in China using environmental and socioeconomic data. BMC Infect Dis. 2016;16(1):343. [Link] [DOI:10.1186/s12879-016-1653-5]
20. Gracie R, Barcellos C, Magalhães M, Souza-Santos R, Guimarães Barrocas PR. Geographical scale effects on the analysis of leptospirosis determinants. Int J Environ Res Public Health. 2014;11(10):10366-83. [Link] [DOI:10.3390/ijerph111010366]
21. Cano-Perez E, Loyola S, Espitia-Almeida F, Torres-Pacheco J, Malambo-Garcıa D, Gomez-Camargo D. Climatic variability and human leptospirosis cases in Cartagena, Colombia: A 10-year ecological study. Am J Trop Med Hyg. 2021;106(3):785-91. [Link] [DOI:10.4269/ajtmh.21-0890]
22. Ferreira MC, Ferreira MFM. Influence of topographic and hydrographic factors on the spatial distribution of leptospirosis disease in São Paulo County, Brazil: An approach using geospatial techniques and GIS analysis. Int Arch Photogramm Remote Sens Spat Inf Sci. 2016;XLI-B8:197-201. [Link] [DOI:10.5194/isprsarchives-XLI-B8-197-2016]
23. Bacallao J, Schneider MC, Najera P, Aldighieri S, Soto A, Marquiño W, et al. Socioeconomic factors and vulnerability to outbreaks of leptospirosis in Nicaragua. Int J Environ Res Public Health. 2014;11(8):8301-18. [Link] [DOI:10.3390/ijerph110808301]
24. Dias JP, Teixeira MG, Costa MCN, Mendes CMC, Guimarães P, Reis MG, et al. Factors associated with Leptospira sp infection in a large urban center in northeastern Brazil. Rev Soc Bras Med Trop. 2007;40(5):499-504. [Link] [DOI:10.1590/S0037-86822007000500002]
25. Gutiérrez JD, Martínez-Vega RA, Botello H, Ruiz-Herrera FJ, Arenas-López LC, Hernandez-Tellez KD. Environmental and socioeconomic determinants of leptospirosis incidence in Colombia. Cad Saude Publica. 2019;35(3):e00118417. [Link] [DOI:10.1590/0102-311x00118417]
26. Yani T. The percentage of leptospirosis deaths in Indonesia is higher than Covid-19 [Internet]. Jakarta: Media Indonesia; 2023 [cited 2025 Mar 4]. Available from: https://mediaindonesia.com/humaniora/564366/persentase-kematian-leptospirosis-di-indonesia-lebih-tinggi-dari-covid-19. [Indonesian] [Link]
27. Dinas Kesehatan Daerah Istimewa Yogyakarta. Health Profile of DI Yogyakarta 2023. Yogyakarta: Dinas Kesehatan DIY; 2023. [Indonesian] [Link]
28. El-Tras WF, Bruce M, Holt HR, Eltholth MM, Merien F. Update on the status of leptospirosis in New Zealand. Acta Trop. 2018;188:161-7. [Link] [DOI:10.1016/j.actatropica.2018.08.021]
29. Puca E, Pipero P, Harxhi A, Abazaj E, Gega A, Puca E, et al. The role of gender in the prevalence of human leptospirosis in Albania. J Infect Dev Ctries. 2018;12(3):150-5. [Link] [DOI:10.3855/jidc.9805]
30. Alavi SM, Khoshkho MM. Seroprevalence study of leptospirosis among rice farmers in Khuzestan province, South west Iran, 2012. Jundishapur J Microbiol. 2014;7(7):e11536. [Link] [DOI:10.5812/jjm.11536]
31. Galan DI, Schneider MC, Roess AA. Leptospirosis risk among occupational groups in Brazil, 2010-2015. Am J Trop Med Hyg. 2023;109(2):376-86. [Link] [DOI:10.4269/ajtmh.21-0181]
32. Mevada Y, Vinod KK, Balamurgan V, Chavhan S, Kumar J, Palkhade R. Seroprevalence and risk factors associated with leptospirosis in high risk occupational groups in the state of gujarat as determined by IgM ELISA and MAT test: A cross sectional study. Indian J Occup Environ Med. 2024;28(2):106-14. [Link] [DOI:10.4103/ijoem.ijoem_83_23]
33. Gancheva G, Doichinova TZ, Karcheva M, Tzvetanova C. Professional risk for leptospirosis. Gen Med. 2014;16(1):25-31. [Link]
34. Colavita G, Paoletti M. Leptospirosis: Occupational risk in the chain of food of animal origin. GIORNALE ITALIANO DI MEDICINA DEL LAVORO ED ERGONOMIA. 2007;29(1):21-4. [Italian] [Link]
35. Narita M, Fujitani S, Haake DA, Paterson DL. Leptospirosis after recreational exposure to water in the Yaeyama Islands, Japan. Am J Trop Med Hyg. 2005;73(4):652-6. [Link] [DOI:10.4269/ajtmh.2005.73.652]
36. Atil A, Jeffree MS, Rahim SSSA, Hassan MR, Lukman KA, Ahmed K. Occupational determinants of leptospirosis among urban service workers. Int J Environ Res Public Health. 2020;17(2):427. [Link] [DOI:10.3390/ijerph17020427]
37. Ayala-Torres JR, Hernández-Morales MF, Alanis-Gallardo VM, Arvizu-Tovar LO, Soberanis-Ramos O. Seroprevalence of leptospirosis in a Mexican military population working with animals. Public Health Chall. 2024;3(2):e193. [Link] [DOI:10.1002/puh2.193]
38. Biswas A, Tiong M, Irvin E, Zhai G, Sinkins M, Johnston H, et al. Gender and sex differences in occupation-specific infectious diseases: A systematic review. Occup Environ Med. 2024;81(8):425-32. [Link] [DOI:10.1136/oemed-2024-109451]
39. Baharom M, Ahmad N, Hod R, Ja'afar MH, Arsad FS, Tangang F, et al. Environmental and occupational factors associated with leptospirosis: A systematic review. Heliyon. 2024;10(1):e23473. [Link] [DOI:10.1016/j.heliyon.2023.e23473]
40. Garvey P, Connell J, O'Flanagan D, McKeown P. Leptospirosis in Ireland: Annual incidence and exposures associated with infection. Epidemiol Infect. 2014;142(4):847-55. [Link] [DOI:10.1017/S0950268813001775]
41. Agampodi SB, Agampodi TC, Thalagala E, Perera S, Chandraratne S, Fernando S. Do people know adequately about leptospirosis? A knowledge assessment survey in post-outbreak situation in Srilanka. Int J Prev Med. 2010;1(3):158-63. [Link]
42. Mohan ARM, Chadee DD. Knowledge, attitudes and practices of Trinidadian households regarding leptospirosis and related matters. Int Health. 2011;3(2):131-7. [Link] [DOI:10.1016/j.inhe.2011.03.002]
43. Oliveira DSC, Guimarães MJB, Portugal JL, Medeiros Z. The socio-demographic, environmental and reservoir factors associated with leptospirosis in an urban area of north-eastern Brazil. Ann Trop Med Parasitol. 2009;103(2):149-57. [Link] [DOI:10.1179/136485909X398221]
44. Sutiningsih D, Sari DP, Permatasari CD, Azzahra NA, Rodriguez-Morales AJ, Yuliawati S, et al. Geospatial analysis of abiotic and biotic conditions associated with leptospirosis in the Klaten Regency, Central Java, Indonesia. Trop Med Infect Dis. 2024;9(10):225. [Link] [DOI:10.3390/tropicalmed9100225]
45. Ifejube OJ, Kuriakose SL, Anish TS, Van Westen C, Blanford JI. Analysing the outbreaks of leptospirosis after floods in Kerala, India. Int J Health Geogr. 2024;23(1):11. [Link] [DOI:10.1186/s12942-024-00372-9]
46. Londe LR, Da Conceição RS, Bernardes T, Dias MCA. Flood-related leptospirosis outbreaks in Brazil: Perspectives for a joint monitoring by health services and disaster monitoring centers. Nat Hazards. 2016;84(2):1419-35. [Link] [DOI:10.1007/s11069-016-2493-8]
47. Gutierrez JD. Effects of meteorological factors on human leptospirosis in Colombia. Int J Biometeorol. 2021;65(2):257-63. [Link] [DOI:10.1007/s00484-020-02028-2]
48. Duarte JL, Giatti LL. Leptospirosis incidence in a state capital in the Western Brazilian Amazon and its relationship with climate and environmental variability, 2008-2013. EPIDEMIOLOGIA E SERVICOS DE SAUDE. 2019;28(1):e2017224. [Link] [DOI:10.5123/S1679-49742019000100009]
49. Nuha NF, Aprilianintyas NA, Novitasari D. Literature review: Relationship of environmental risk factors and the incidence of leptospirosis in settlements (2018-2023). J Environ Health. 2023;15(3):235-46. [Link] [DOI:10.20473/jkl.v15i3.2023.235-246]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






