Volume 12, Issue 3 (2024)
Health Educ Health Promot 2024, 12(3): 495-503 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Supriadi S, Naswir M, Johari A, Fahri S. Risk Factors for Scabies among Schoolchildren in Low- and Middle-Income Countries. Health Educ Health Promot 2024; 12 (3) :495-503
URL: http://hehp.modares.ac.ir/article-5-76515-en.html
URL: http://hehp.modares.ac.ir/article-5-76515-en.html
1- Department of Environmental Health, Health Polytechnic of the Ministry of Health Jambi, Jambi, Indonesia
2- Department of Public Health Education, Faculty of MIPA Doctoral Program, Jambi University, Jambi, Indonesia
2- Department of Public Health Education, Faculty of MIPA Doctoral Program, Jambi University, Jambi, Indonesia
Full-Text [PDF 646 kb]
(2545 Downloads)
| Abstract (HTML) (1464 Views)
Full-Text: (267 Views)
Introduction
Infections of the skin and soft tissues represent a major global health concern. While not life-threatening, conditions, like scabies are among the most prevalent itchy skin disorders worldwide and significantly contribute to the overall disease burden [1]. Scabies is categorized as a neglected tropical disease by the World Health Organization (WHO) and is included on its roster of such diseases [2, 3].
Scabies is a global issue, impacting an estimated 200 million individuals at any given time [4]. Studies reveal that scabies prevalence is highest in tropical regions of East Asia, Southeast Asia, Oceania, and Tropical Latin America. This condition accounts for 0.21% of global disability-adjusted life years (DALYs) [5]. Despite its prevalence, there are significant gaps in our understanding of the epidemiology and risk factors of scabies, particularly in regions with a high disease burden. For instance, in Malaysia, the most recent study on scabies in children was conducted in 1980, and the subsequent study took place in nursing homes in 2010 [6].
Scabies is triggered by the mite Sarcoptes scabiei, which invades the outer layer of the skin, causing intense itching and scratching due to allergic reactions to the mites’ proteins and waste. It spreads primarily through direct skin contact. While it frequently affects vulnerable populations, such as young children and the elderly, anyone, regardless of age, gender, or hygiene level, can be affected. Complications from scabies can include secondary bacterial infections, septicemia, rheumatic fever, and post-streptococcal glomerulonephritis [7].
Scabies alone is responsible for about 0.21% of global DALYs across all health conditions [6]. Estimates of scabies prevalence worldwide vary widely, ranging from 0.2% to 71% [3]. This condition is widespread in many impoverished tropical areas, affecting 5-10% of children. Frequent recurrences of scabies place a significant strain on healthcare systems due to both the infestations and their associated complications [8]. Factors contributing to scabies include low socioeconomic status, high population density, inadequate water management, and poor sanitation [8, 9]. Scabies is strongly linked to personal hygiene and living conditions [10], making it common among people who reside in crowded environments, such as densely populated areas or boarding schools [11, 12]. Outbreaks of scabies frequently occur in settings where there is close and prolonged skin-to-skin contact, including daycare centers, orphanages, elderly care facilities, prisons, refugee camps, Islamic boarding schools, and even hospitals [13]. Most scabies cases are found in low-income countries, often due to limited awareness, insufficient preventive measures, and inadequate facilities [6]. Additionally, scabies has been shown to negatively impact children’s learning in school [14].
The treatment of scabies is relatively costly, particularly as it often affects individuals from low-income backgrounds who struggle to afford healthcare expenses [15]. The costs can become even higher when the patient suffers from severe scabies, especially if there are complications due to secondary bacterial infections [16]. At the household level, money spent on medical care can reduce the budget available for essential needs like food, placing an additional financial strain on the family. At the institutional level, substantial resources are required to manage scabies outbreaks effectively [17].
The previous systematic review did not clearly define comparisons between countries and the types of schools [18]. It is very important to understand the differences in these situations as a basis for determining interventions more suitable to each country’s conditions. This review focused on uncovering the risk factors for scabies among school-aged children in low- and middle-income countries (LMICs).
Information and Methods
Protocol
This systematic review analyzed the literature on risk factors related to the incidence of scabies in school students, particularly in LMICs published from 2000 to 2023 based on the World Bank list. The systematic steps included developing research questions using patient, intervention, comparison, outcome (PICO) structure, searching for relevant articles, assessing article eligibility, extracting data, assessing quality, documenting results, and summarizing findings in a narrative analysis [19].
Search strategy
The search for relevant articles utilized online database search engines, including ScienceDirect, PubMed, JSTOR, and ProQuest, within the publication period from 2000 to 2023. The search keyword arrangement used Boolean operators with the following structure: "Scabies" OR "Sarcoptes scabiei" AND "Risk factors" OR "Predictive factors" AND "School-children" OR "student" OR "Boarding-school" OR "Public school".
Inclusion and exclusion criteria
The studies selected for this review were chosen according to the criteria, which covered aspects, such as participant characteristics, intervention specifics, comparison groups, outcomes, and study design (PICO). This search focused on students from the community, dormitory, and elementary schools. The inclusion criteria were quantitative research that matched the search keywords, availability of full-text articles, original research papers, and articles written in English. Studies were excluded if they were: case studies, review articles with an unusual narrative ratio, non-peer-reviewed papers or theses, or articles focusing on general skin infection outcomes. Initially, articles were screened based on their titles and abstracts. Only selected full-text and eligible articles were included in the qualitative analysis.
Data extraction
Each author compiled data from the articles into a structured extraction table, with columns for details, such as article number, name of author(a), title, year of study, objective, methodology, parameters studied, findings, and themes. The second reviewer subsequently examined the assigned articles, validated the information, and added further comments to the table.
Assessment of evidence quality
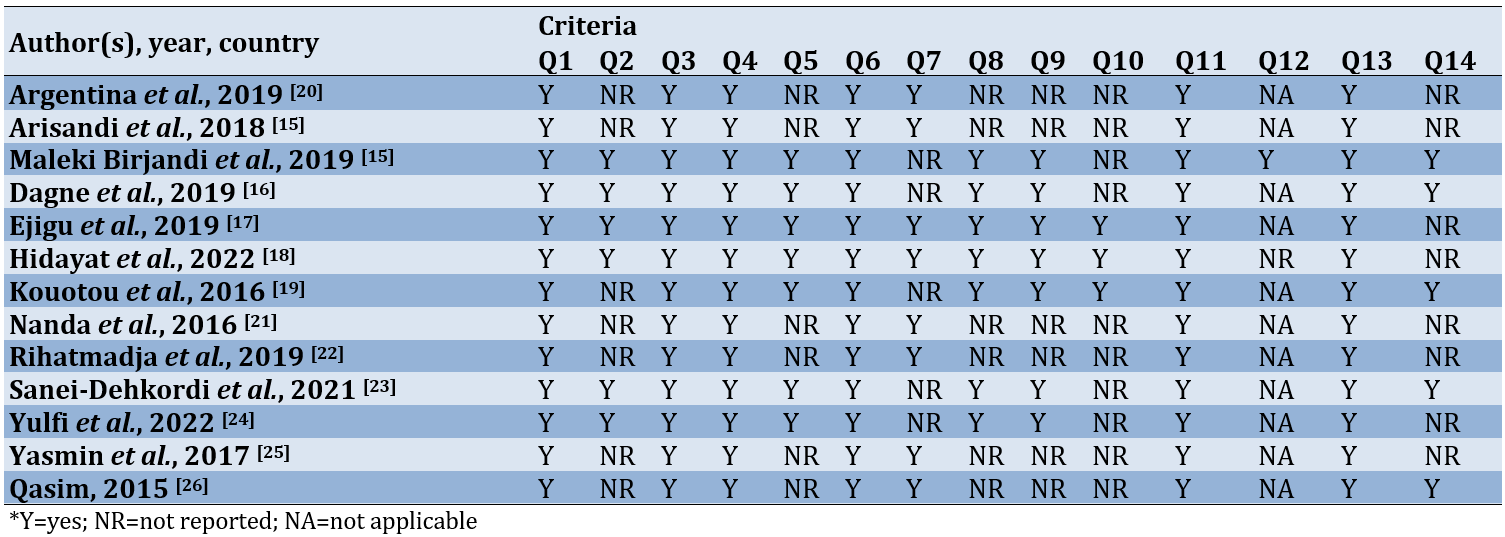
The author independently evaluated the quality of each eligible study. This evaluation utilized a tool provided by the National Institutes of Health (NIH) specifically designed for observational cohort and cross-sectional studies. The tool included 14 questions that serve as criteria for determining the study’s quality, focusing on the core concept of internal validity. The categorization of study quality consisted of three levels, including good (90-100%), fair (70-89%), and poor (≤69%).
Findings
Study selection
The database search yielded a total of 448 articles, including those found through manual searching. After removing duplicates and reviewing titles and abstracts, 68 articles were selected for further screening. Out of these, 41 articles were excluded for reasons, such as focusing on a different population as the research subject, being non-English, being irrelevant to the research, or relying on hospital data. The final selection resulted in 13 studies being included in the systematic review (Figure 1, Table 1).
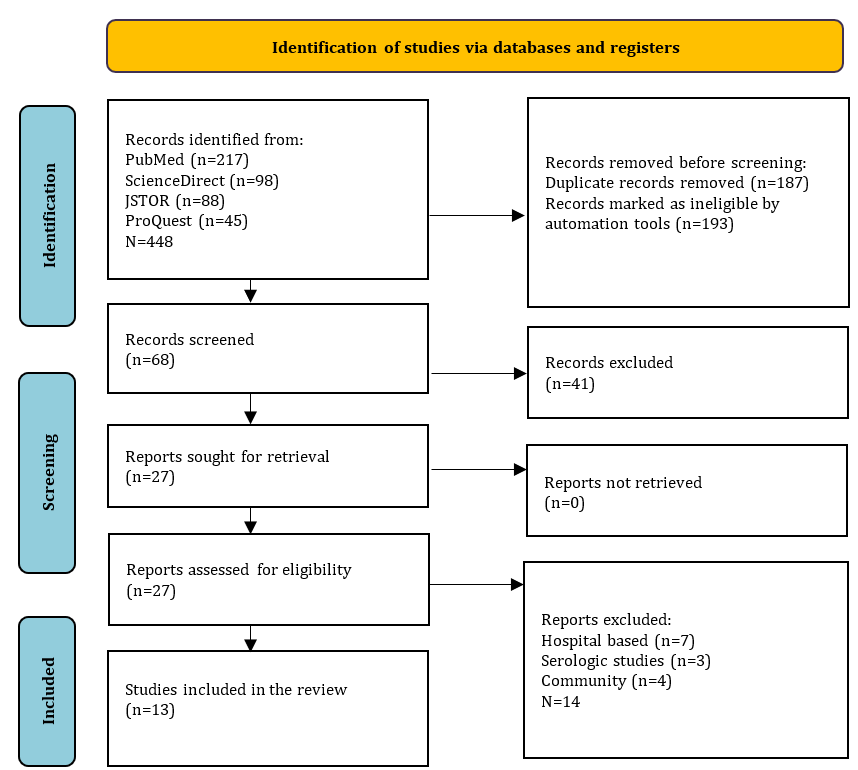
Figure 1. PRISMA flowchart for literature search.
Table 1. Data extraction of the included studies
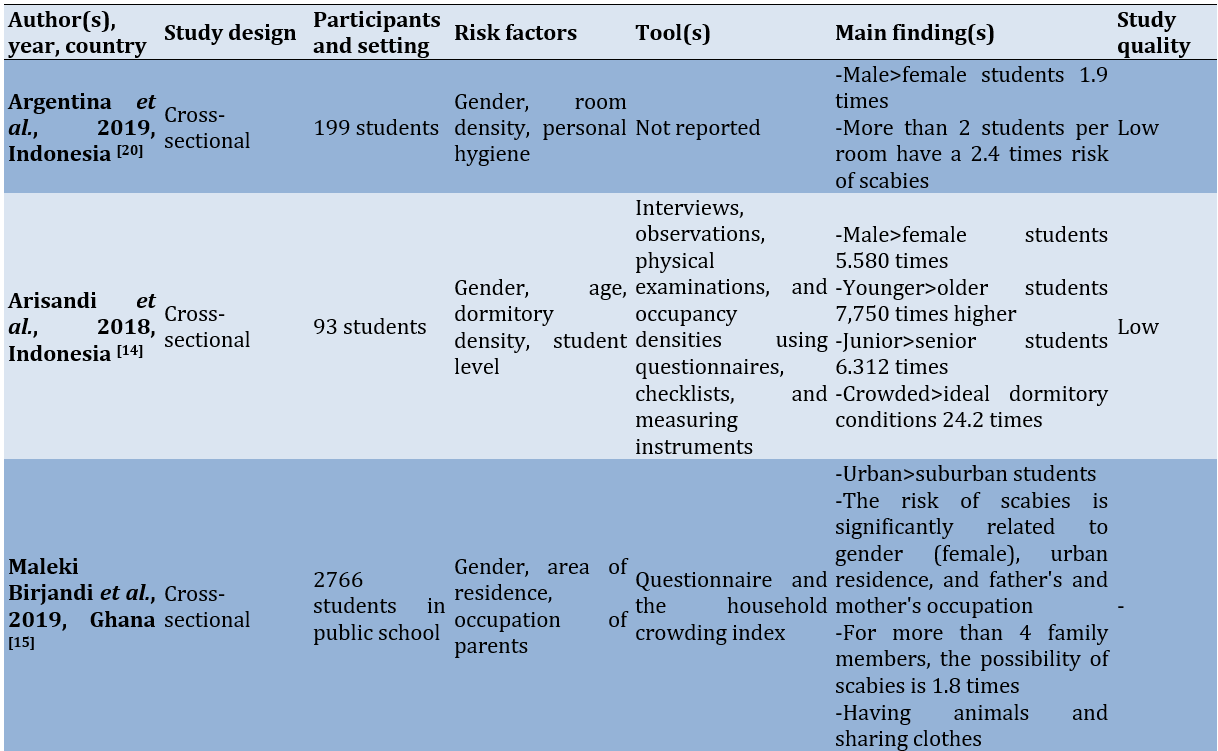
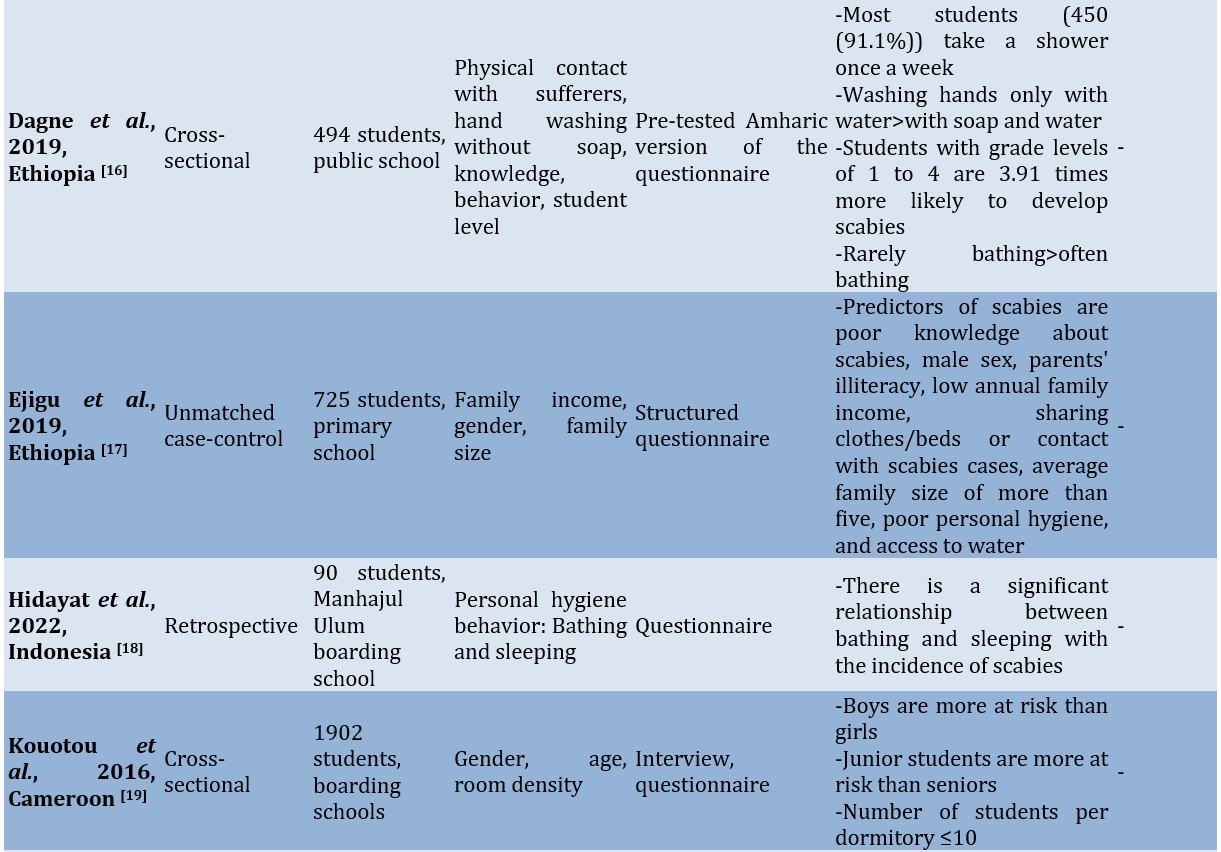
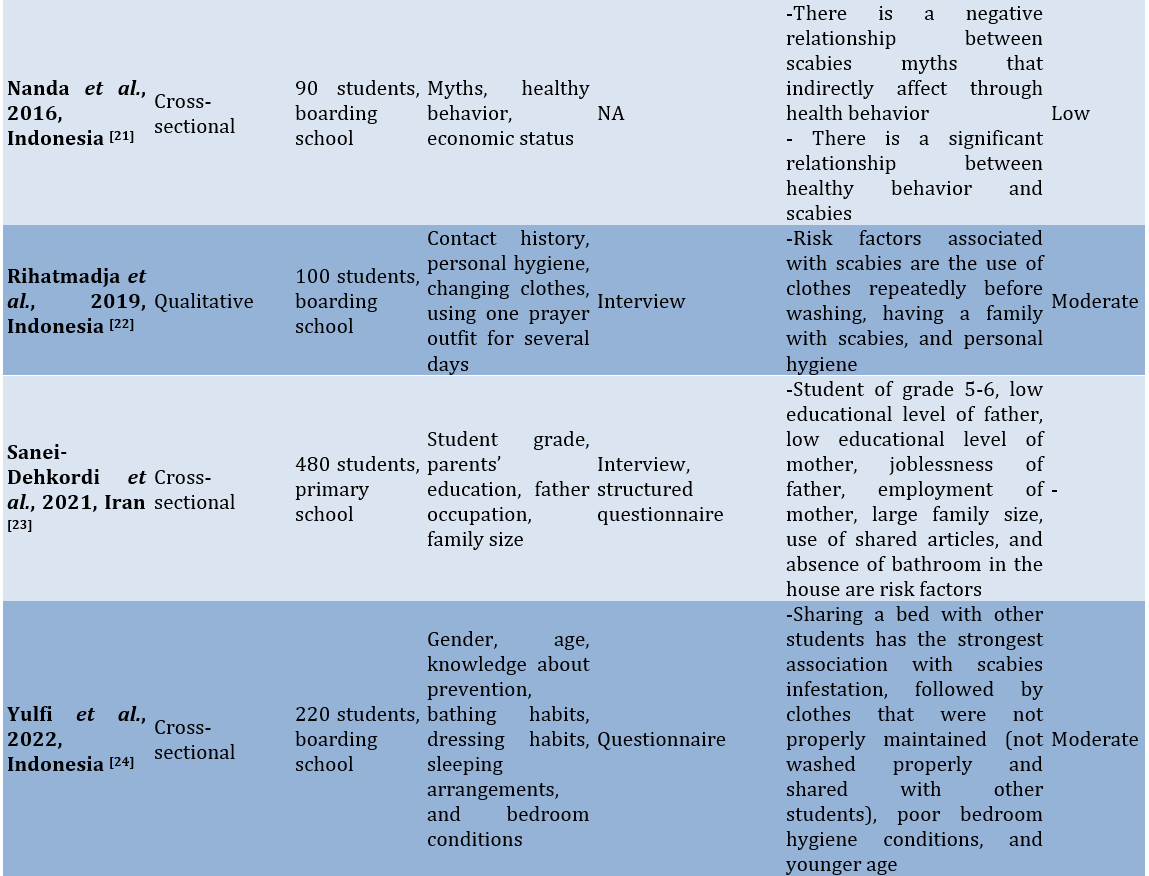
Study quality
According to the evaluation results from the NIH quality assessment tool for observational cohort and cross-sectional studies, which examines 14 criteria, six studies were rated as “poor” [21-26], while seven others were classified as “Fair” (Table 2) [14, 15, 21, 22, 25, 26, 27,].
Table 2. Summary of quality assessment
Risk factors for scabies in school-aged children
Several risk factors for scabies included gender, age, dormitory density, student level, physical contact with sufferers, family income, parental occupation, knowledge, hand-washing behavior, the number of family members living at home, personal hygiene, exchanging clothes, using clothes repeatedly without washing, bathing habits, characteristics of the house, pets, and the frequency of changing bed linens.
There were differences in risk factors between students in dormitories and those in public schools. Risk factors identified for students living in dormitories included dormitory density (or the density of occupants in each bedroom), changing clothes, and using clothes repeatedly without washing. In contrast, the risk factors for scabies found among students attending public schools included the number of family members living in the same household, the characteristics of the house, the area where they live, pets, and the frequency of changing clothes. Meanwhile, the similarities in risk factors between the two situations include age, gender, student level, personal hygiene, frequency of changing bed sheets, and family economic status.
Gender, age, and student grade as the risk factors for scabies
Gender differences appeared to be one of the risk factors for scabies. This is not necessarily directly related to hormonal or genetic factors. Instead, the behavior of men and women in their daily lives was the main focus discussed in the analyzed studies. In Indonesia, there is a habit of wearing the same clothes repeatedly without washing them [16]. In religious boarding schools (Pesantren), students regularly pray five times a day using special clothes commonly worn by male Muslims (Baju Koko); sometimes, these clothes are also worn when male students attend class [17]. In contrast, female students typically wear a special garment designed for prayer, commonly called “Mukena,” which is worn only during prayer [18]. Male students generally pay less attention to their appearance and personal hygiene than female students [19]. This situation is markedly different from that of female students living in dormitories in Ghana [23], where they rarely bathe or change clothes, often sleep on the floor, and seldom sleep alone.
Age and student grade also contributed to the risk of scabies among students, with younger individuals and juniors in school being more likely to contract scabies compared to older individuals and seniors [18]. This is due to the lack of independence and knowledge that juniors have regarding personal hygiene and the maintenance of their surrounding environment [16]. Studies in boarding schools in Cameroon indicate that younger students (<15 years old) tend to exchange clothes and beds [16].
Different situation of students in public vs. boarding schools
Family circumstances play a crucial role in the risk of scabies among students. In LMICs, poverty is a major issue closely related to family health conditions. The lack of family income seems to impact the ability to own a home, making it common for many family members to live together [24]. Students who attend public schools and live with their parents often experience scabies due to crowding, physical contact with other family members, a lack of parental attention to personal hygiene, and a high-risk home environment. The presence of pets in the home also contributes to the incidence of scabies [24]. Meanwhile, students living in school dormitories frequently encounter crowded conditions due to the high number of residents, resulting in close interactions among them [24, 28]. Common practices, such as sharing beds, exchanging clothes, and using the same toiletries are primary causes of scabies outbreaks [29, 30]. It appears that dormitory caregivers do not adequately address the students’ hygiene needs, which require attention typically provided by their parents at home [31].
Differences in risk factors among low- and middle-income countries
In studies conducted in Indonesia, most were situated in dormitories or Islamic boarding schools. Common risk factors identified included the frequent exchange of clothes among room occupants, the repeated use of prayer garments without washing them first, and the disorganized condition of the rooms. One study noted that there is a myth in the community regarding the conditions in Islamic boarding schools, suggesting that they are prone to skin diseases, which affects health behavior [14].
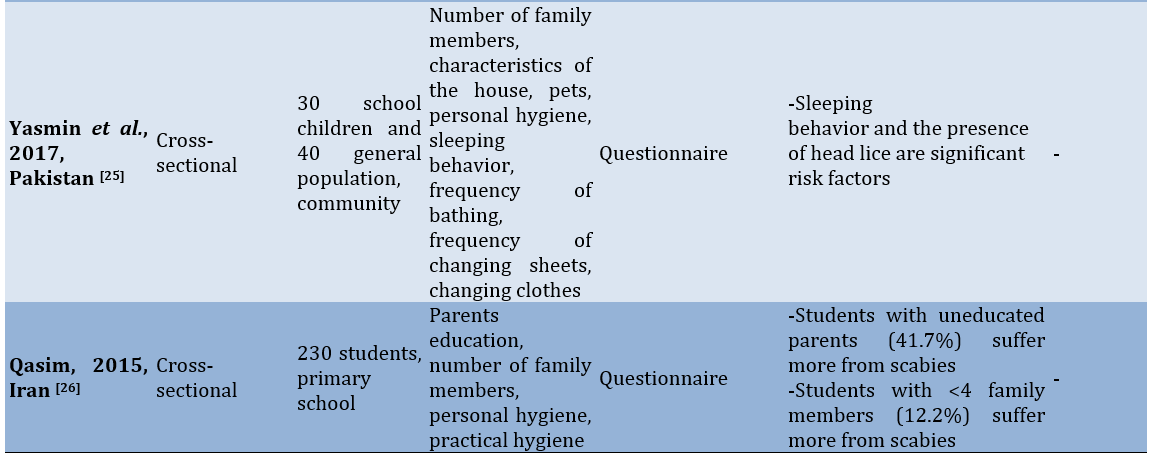
In countries on the African continent, such as Ghana, Ethiopia, and Cameroon, risk factors for scabies include the area of residence, bathing frequency, handwashing habits without the use of soap, and parents’ occupations. Studies in Iran indicate that the availability of bathrooms is a risk factor for scabies. Meanwhile, a study in Pakistan identified pets and the characteristics of the house as risk factors for scabies [15].
Discussion
The present review assessed the risk factors associated with scabies in school students in LMICs. Thirteen studies were identified from various databases without a time frame filter. Most studies were from Asia, while some were from Africa. Geographically, there are differences between these countries; however, regarding economic status, similarities relate to the community’s ability to maintain healthy behavior. There are notable differences among the countries included in this review.
Demographic factors are the most prominent risk factors compared to others, as these demographic factors appear in almost all of the studies included in this review. Gender and age are the most important demographic factors to consider. All included studies agreed that male students are the most dominant group with a higher potential to experience scabies compared to female students. Only one study in Ghana found that women are at greater risk of experiencing scabies than men [27]. Boys are more likely to sleep together, share clothing, and have close contact with girls, which increases their risk of scabies [16]. Research indicates that sex differences in scabies risk are influenced by personal habits, such as how often one bathes or changes clothes; sleeping on the floor raises the risk of infection, while sleeping alone lowers it [21]. Normaznah et al. [29] conducted a serological survey of indigenous populations in Malaysia, reporting no significant differences in scabies incidence between the sexes [22-24]. This suggests that the characteristics of the study population and the research methods used can affect the results. Regarding age, the incidence of scabies declines with increasing age, which aligns with earlier studies [25, 26]. The condition is most prevalent among public school students because younger children frequently have close physical contact with many family members, increasing the likelihood of exposure to scabies-infected relatives [27]. In Iran, younger or lower-grade students are more susceptible to scabies than their older peers [19]. This can be attributed to the more frequent physical interactions among students of similar ages. Other studies have highlighted the significant role of physical contact in the transmission of scabies [14].
In Indonesian Islamic boarding schools, there is a common belief that students are more prone to skin diseases. Rihatmadja et al. [22] discovered that students who own only one or two “Koko clothes” often wear them repeatedly without washing, as they believe it is necessary to wear Koko clothes during prayers or when meeting their teacher. This practice is also observed among female students, who frequently use “Mukena” in a similar manner [24]. In the Pesantren, students often share personal items, including towels, which increases the spread of scabies among students living in Islamic boarding schools [32]. The family’s economic situation also contributes to the prevalence of scabies in Islamic boarding schools, with students from families with better economic status being less likely to experience scabies than those from families with lower economic status. The provision of personal hygiene equipment is influenced by the economic conditions of the students’ families; however, it is not the main factor in the prevalence of scabies.
Housing conditions, such as limited access to clean water, living in homes made of soft bricks, and keeping animals indoors, can elevate the risk of scabies infection. This review revealed inconsistent findings regarding whether owning pets raises or lowers the risk of scabies. The impact of pet ownership may vary depending on other factors, such as the population density of the area. Having animals indoors can exacerbate overcrowding and hygiene issues, thereby heightening the risk of scabies infestation. Conversely, if animals reside in large homes with separate sleeping areas from humans, the likelihood of scabies infestation decreases. A lack of water or poor water usage is also recognized as a risk factor for scabies. These findings are consistent with earlier research conducted in northern Ethiopia [33].
The factors influencing the risk of scabies vary between rural and urban environments. Previous studies suggest that individuals in rural and suburban areas generally reside in less crowded spaces, while those in urban areas face higher population density, which increases the likelihood of disease transmission through close contact. Urban issues, such as high density, migration, and displacement exacerbate the prevalence of scabies. Conversely, other research challenges this view, proposing that scabies might be more common in rural areas due to inadequate socioeconomic conditions and restricted access to healthcare [34, 35]. Additionally, access to a clean water supply is a factor in the prevalence of scabies. A study in Ethiopia indicates that the frequency of bathing among students is once a week, with some students bathing more than once a week. It was also noted that there are behaviors less related to personal hygiene, such as washing hands without using soap, which contributes to the prevalence of scabies often found on students’ arms [14].
Participants in a mixed-method study reported that they sometimes missed classes due to the distracting itching, which hindered their ability to concentrate on their studies [36]. Feelings of inferiority do not seem to significantly affect class attendance. However, friendships may become strained as students avoid physical contact, such as handshakes, to prevent the transmission of scabies [36]. This situation supports findings from previous studies in Indonesia, which show a significant relationship between scabies incidence and students’ concentration in learning, as measured by the Brand Aufgaben test [37]. Studies in Brazil indicate that the concentration and enthusiasm of students with scabies are also affected by weakened physical conditions due to poor sleep quality, as frequent waking from itching disrupts their rest [38]. Decreased sleep quality can impact a person’s ability to concentrate, make decisions, and participate in activities. If this condition persists, it can ultimately lead to a decline in academic performance [39]. In the Bijagós community, scabies is heavily stigmatized due to concerns about its contagious nature. This stigma is linked to discriminatory attitudes, feelings of rejection, social isolation, and specific behaviors related to seeking treatment. The delay in seeking medical help and the resulting health issues may be exacerbated by the stigma surrounding the condition. Thus, students in the community may feel deeply affected and embarrassed to attend class [40].
Considering the risk factors prevalent in LMICs, both individual and environmental hygiene issues significantly contribute to the overall risk profile. Additionally, most communities in LMICs live below the poverty line, a condition closely linked to inadequate attention to hygiene [41, 42]. Environmental management appears to be a promising intervention to reduce the incidence of scabies. For instance, a boarding school in Indonesia implemented an intensive cleaning campaign over three consecutive weeks, leading to improved hygiene practices among the students. However, such campaigns alone are insufficient; they must be complemented by comprehensive health education on scabies [43]. Lopes et al. [44] conducted educational activities in Nigeria among boarding school students under 11 years old, using a learning module presented by a dermatologist with engaging PowerPoint slides. The results are positive, showing increased knowledge and behavioral changes among the students. Nevertheless, it is crucial for the government to ensure that this educational program is implemented routinely for optimal results, and it should not be prohibitively expensive.
A study in Bangladesh implemented a four-month program to control scabies in an Islamic boarding school. The program include daily monitoring of personal hygiene, weekly health education classes, and monthly meetings between class monitors and scabies control officers. As a result, the school leadership, dormitory caregivers, and all students are fully committed to maintaining personal hygiene and the cleanliness of the dormitory environment [45].
One of the limitations of this study is the quality of the studies. Most included studies were of low or fair quality, raising concerns about the generalizability of the findings. Also, limited access to high-quality journals may have skewed the results toward studies published in less reputable sources. The majority of studies originated from Asia, with only a few from Africa. This limits the generalizability to other LMICs. Therefore, caution should be exercised when using the results of these studies.
This study identified several risk factors with strong significance for the incidence of scabies in children in LMICs. Additionally, this study identified differences in risk factors between students who attended public schools and those in boarding schools or Islamic boarding schools. To effectively prevent and control scabies in children, public health initiatives must consider a comprehensive range of contributing factors. This can be achieved through multisectoral strategies and partnerships that address all the elements involved in scabies transmission.
Conclusion
Gender, age, family economic status, and personal hygiene are risk factors for scabies in schoolchildren in LMICs.
Acknowledgments: We would like to express our gratitude to the Head of Jambi Health Polytechnic who provided material support in the implementation of this study.
Ethical Permissions: Not applicable.
Conflicts of Interests: The authors declared no conflicts of interests.
Authors' Contribution: Supriadi S (First Author), Introduction Writer/Main Researcher/Methodologist/Discussion Writer/Statistical Analyst (40%); Naswir M (Second Author), Assistant Researcher/Discussion Writer (20%); Johari A (Third Author), Introduction Writer/Assistant Researcher/Discussion Writer (20%); Fahri S (Fourth Author), Introduction Writer/Assistant Researcher/Discussion Writer (20%)
Funding/Support: This research received no external funding.
Infections of the skin and soft tissues represent a major global health concern. While not life-threatening, conditions, like scabies are among the most prevalent itchy skin disorders worldwide and significantly contribute to the overall disease burden [1]. Scabies is categorized as a neglected tropical disease by the World Health Organization (WHO) and is included on its roster of such diseases [2, 3].
Scabies is a global issue, impacting an estimated 200 million individuals at any given time [4]. Studies reveal that scabies prevalence is highest in tropical regions of East Asia, Southeast Asia, Oceania, and Tropical Latin America. This condition accounts for 0.21% of global disability-adjusted life years (DALYs) [5]. Despite its prevalence, there are significant gaps in our understanding of the epidemiology and risk factors of scabies, particularly in regions with a high disease burden. For instance, in Malaysia, the most recent study on scabies in children was conducted in 1980, and the subsequent study took place in nursing homes in 2010 [6].
Scabies is triggered by the mite Sarcoptes scabiei, which invades the outer layer of the skin, causing intense itching and scratching due to allergic reactions to the mites’ proteins and waste. It spreads primarily through direct skin contact. While it frequently affects vulnerable populations, such as young children and the elderly, anyone, regardless of age, gender, or hygiene level, can be affected. Complications from scabies can include secondary bacterial infections, septicemia, rheumatic fever, and post-streptococcal glomerulonephritis [7].
Scabies alone is responsible for about 0.21% of global DALYs across all health conditions [6]. Estimates of scabies prevalence worldwide vary widely, ranging from 0.2% to 71% [3]. This condition is widespread in many impoverished tropical areas, affecting 5-10% of children. Frequent recurrences of scabies place a significant strain on healthcare systems due to both the infestations and their associated complications [8]. Factors contributing to scabies include low socioeconomic status, high population density, inadequate water management, and poor sanitation [8, 9]. Scabies is strongly linked to personal hygiene and living conditions [10], making it common among people who reside in crowded environments, such as densely populated areas or boarding schools [11, 12]. Outbreaks of scabies frequently occur in settings where there is close and prolonged skin-to-skin contact, including daycare centers, orphanages, elderly care facilities, prisons, refugee camps, Islamic boarding schools, and even hospitals [13]. Most scabies cases are found in low-income countries, often due to limited awareness, insufficient preventive measures, and inadequate facilities [6]. Additionally, scabies has been shown to negatively impact children’s learning in school [14].
The treatment of scabies is relatively costly, particularly as it often affects individuals from low-income backgrounds who struggle to afford healthcare expenses [15]. The costs can become even higher when the patient suffers from severe scabies, especially if there are complications due to secondary bacterial infections [16]. At the household level, money spent on medical care can reduce the budget available for essential needs like food, placing an additional financial strain on the family. At the institutional level, substantial resources are required to manage scabies outbreaks effectively [17].
The previous systematic review did not clearly define comparisons between countries and the types of schools [18]. It is very important to understand the differences in these situations as a basis for determining interventions more suitable to each country’s conditions. This review focused on uncovering the risk factors for scabies among school-aged children in low- and middle-income countries (LMICs).
Information and Methods
Protocol
This systematic review analyzed the literature on risk factors related to the incidence of scabies in school students, particularly in LMICs published from 2000 to 2023 based on the World Bank list. The systematic steps included developing research questions using patient, intervention, comparison, outcome (PICO) structure, searching for relevant articles, assessing article eligibility, extracting data, assessing quality, documenting results, and summarizing findings in a narrative analysis [19].
Search strategy
The search for relevant articles utilized online database search engines, including ScienceDirect, PubMed, JSTOR, and ProQuest, within the publication period from 2000 to 2023. The search keyword arrangement used Boolean operators with the following structure: "Scabies" OR "Sarcoptes scabiei" AND "Risk factors" OR "Predictive factors" AND "School-children" OR "student" OR "Boarding-school" OR "Public school".
Inclusion and exclusion criteria
The studies selected for this review were chosen according to the criteria, which covered aspects, such as participant characteristics, intervention specifics, comparison groups, outcomes, and study design (PICO). This search focused on students from the community, dormitory, and elementary schools. The inclusion criteria were quantitative research that matched the search keywords, availability of full-text articles, original research papers, and articles written in English. Studies were excluded if they were: case studies, review articles with an unusual narrative ratio, non-peer-reviewed papers or theses, or articles focusing on general skin infection outcomes. Initially, articles were screened based on their titles and abstracts. Only selected full-text and eligible articles were included in the qualitative analysis.
Data extraction
Each author compiled data from the articles into a structured extraction table, with columns for details, such as article number, name of author(a), title, year of study, objective, methodology, parameters studied, findings, and themes. The second reviewer subsequently examined the assigned articles, validated the information, and added further comments to the table.
Assessment of evidence quality
The author independently evaluated the quality of each eligible study. This evaluation utilized a tool provided by the National Institutes of Health (NIH) specifically designed for observational cohort and cross-sectional studies. The tool included 14 questions that serve as criteria for determining the study’s quality, focusing on the core concept of internal validity. The categorization of study quality consisted of three levels, including good (90-100%), fair (70-89%), and poor (≤69%).
Findings
Study selection
The database search yielded a total of 448 articles, including those found through manual searching. After removing duplicates and reviewing titles and abstracts, 68 articles were selected for further screening. Out of these, 41 articles were excluded for reasons, such as focusing on a different population as the research subject, being non-English, being irrelevant to the research, or relying on hospital data. The final selection resulted in 13 studies being included in the systematic review (Figure 1, Table 1).

Figure 1. PRISMA flowchart for literature search.
Table 1. Data extraction of the included studies




Study quality
According to the evaluation results from the NIH quality assessment tool for observational cohort and cross-sectional studies, which examines 14 criteria, six studies were rated as “poor” [21-26], while seven others were classified as “Fair” (Table 2) [14, 15, 21, 22, 25, 26, 27,].
Table 2. Summary of quality assessment

Risk factors for scabies in school-aged children
Several risk factors for scabies included gender, age, dormitory density, student level, physical contact with sufferers, family income, parental occupation, knowledge, hand-washing behavior, the number of family members living at home, personal hygiene, exchanging clothes, using clothes repeatedly without washing, bathing habits, characteristics of the house, pets, and the frequency of changing bed linens.
There were differences in risk factors between students in dormitories and those in public schools. Risk factors identified for students living in dormitories included dormitory density (or the density of occupants in each bedroom), changing clothes, and using clothes repeatedly without washing. In contrast, the risk factors for scabies found among students attending public schools included the number of family members living in the same household, the characteristics of the house, the area where they live, pets, and the frequency of changing clothes. Meanwhile, the similarities in risk factors between the two situations include age, gender, student level, personal hygiene, frequency of changing bed sheets, and family economic status.
Gender, age, and student grade as the risk factors for scabies
Gender differences appeared to be one of the risk factors for scabies. This is not necessarily directly related to hormonal or genetic factors. Instead, the behavior of men and women in their daily lives was the main focus discussed in the analyzed studies. In Indonesia, there is a habit of wearing the same clothes repeatedly without washing them [16]. In religious boarding schools (Pesantren), students regularly pray five times a day using special clothes commonly worn by male Muslims (Baju Koko); sometimes, these clothes are also worn when male students attend class [17]. In contrast, female students typically wear a special garment designed for prayer, commonly called “Mukena,” which is worn only during prayer [18]. Male students generally pay less attention to their appearance and personal hygiene than female students [19]. This situation is markedly different from that of female students living in dormitories in Ghana [23], where they rarely bathe or change clothes, often sleep on the floor, and seldom sleep alone.
Age and student grade also contributed to the risk of scabies among students, with younger individuals and juniors in school being more likely to contract scabies compared to older individuals and seniors [18]. This is due to the lack of independence and knowledge that juniors have regarding personal hygiene and the maintenance of their surrounding environment [16]. Studies in boarding schools in Cameroon indicate that younger students (<15 years old) tend to exchange clothes and beds [16].
Different situation of students in public vs. boarding schools
Family circumstances play a crucial role in the risk of scabies among students. In LMICs, poverty is a major issue closely related to family health conditions. The lack of family income seems to impact the ability to own a home, making it common for many family members to live together [24]. Students who attend public schools and live with their parents often experience scabies due to crowding, physical contact with other family members, a lack of parental attention to personal hygiene, and a high-risk home environment. The presence of pets in the home also contributes to the incidence of scabies [24]. Meanwhile, students living in school dormitories frequently encounter crowded conditions due to the high number of residents, resulting in close interactions among them [24, 28]. Common practices, such as sharing beds, exchanging clothes, and using the same toiletries are primary causes of scabies outbreaks [29, 30]. It appears that dormitory caregivers do not adequately address the students’ hygiene needs, which require attention typically provided by their parents at home [31].
Differences in risk factors among low- and middle-income countries
In studies conducted in Indonesia, most were situated in dormitories or Islamic boarding schools. Common risk factors identified included the frequent exchange of clothes among room occupants, the repeated use of prayer garments without washing them first, and the disorganized condition of the rooms. One study noted that there is a myth in the community regarding the conditions in Islamic boarding schools, suggesting that they are prone to skin diseases, which affects health behavior [14].
In countries on the African continent, such as Ghana, Ethiopia, and Cameroon, risk factors for scabies include the area of residence, bathing frequency, handwashing habits without the use of soap, and parents’ occupations. Studies in Iran indicate that the availability of bathrooms is a risk factor for scabies. Meanwhile, a study in Pakistan identified pets and the characteristics of the house as risk factors for scabies [15].
Discussion
The present review assessed the risk factors associated with scabies in school students in LMICs. Thirteen studies were identified from various databases without a time frame filter. Most studies were from Asia, while some were from Africa. Geographically, there are differences between these countries; however, regarding economic status, similarities relate to the community’s ability to maintain healthy behavior. There are notable differences among the countries included in this review.
Demographic factors are the most prominent risk factors compared to others, as these demographic factors appear in almost all of the studies included in this review. Gender and age are the most important demographic factors to consider. All included studies agreed that male students are the most dominant group with a higher potential to experience scabies compared to female students. Only one study in Ghana found that women are at greater risk of experiencing scabies than men [27]. Boys are more likely to sleep together, share clothing, and have close contact with girls, which increases their risk of scabies [16]. Research indicates that sex differences in scabies risk are influenced by personal habits, such as how often one bathes or changes clothes; sleeping on the floor raises the risk of infection, while sleeping alone lowers it [21]. Normaznah et al. [29] conducted a serological survey of indigenous populations in Malaysia, reporting no significant differences in scabies incidence between the sexes [22-24]. This suggests that the characteristics of the study population and the research methods used can affect the results. Regarding age, the incidence of scabies declines with increasing age, which aligns with earlier studies [25, 26]. The condition is most prevalent among public school students because younger children frequently have close physical contact with many family members, increasing the likelihood of exposure to scabies-infected relatives [27]. In Iran, younger or lower-grade students are more susceptible to scabies than their older peers [19]. This can be attributed to the more frequent physical interactions among students of similar ages. Other studies have highlighted the significant role of physical contact in the transmission of scabies [14].
In Indonesian Islamic boarding schools, there is a common belief that students are more prone to skin diseases. Rihatmadja et al. [22] discovered that students who own only one or two “Koko clothes” often wear them repeatedly without washing, as they believe it is necessary to wear Koko clothes during prayers or when meeting their teacher. This practice is also observed among female students, who frequently use “Mukena” in a similar manner [24]. In the Pesantren, students often share personal items, including towels, which increases the spread of scabies among students living in Islamic boarding schools [32]. The family’s economic situation also contributes to the prevalence of scabies in Islamic boarding schools, with students from families with better economic status being less likely to experience scabies than those from families with lower economic status. The provision of personal hygiene equipment is influenced by the economic conditions of the students’ families; however, it is not the main factor in the prevalence of scabies.
Housing conditions, such as limited access to clean water, living in homes made of soft bricks, and keeping animals indoors, can elevate the risk of scabies infection. This review revealed inconsistent findings regarding whether owning pets raises or lowers the risk of scabies. The impact of pet ownership may vary depending on other factors, such as the population density of the area. Having animals indoors can exacerbate overcrowding and hygiene issues, thereby heightening the risk of scabies infestation. Conversely, if animals reside in large homes with separate sleeping areas from humans, the likelihood of scabies infestation decreases. A lack of water or poor water usage is also recognized as a risk factor for scabies. These findings are consistent with earlier research conducted in northern Ethiopia [33].
The factors influencing the risk of scabies vary between rural and urban environments. Previous studies suggest that individuals in rural and suburban areas generally reside in less crowded spaces, while those in urban areas face higher population density, which increases the likelihood of disease transmission through close contact. Urban issues, such as high density, migration, and displacement exacerbate the prevalence of scabies. Conversely, other research challenges this view, proposing that scabies might be more common in rural areas due to inadequate socioeconomic conditions and restricted access to healthcare [34, 35]. Additionally, access to a clean water supply is a factor in the prevalence of scabies. A study in Ethiopia indicates that the frequency of bathing among students is once a week, with some students bathing more than once a week. It was also noted that there are behaviors less related to personal hygiene, such as washing hands without using soap, which contributes to the prevalence of scabies often found on students’ arms [14].
Participants in a mixed-method study reported that they sometimes missed classes due to the distracting itching, which hindered their ability to concentrate on their studies [36]. Feelings of inferiority do not seem to significantly affect class attendance. However, friendships may become strained as students avoid physical contact, such as handshakes, to prevent the transmission of scabies [36]. This situation supports findings from previous studies in Indonesia, which show a significant relationship between scabies incidence and students’ concentration in learning, as measured by the Brand Aufgaben test [37]. Studies in Brazil indicate that the concentration and enthusiasm of students with scabies are also affected by weakened physical conditions due to poor sleep quality, as frequent waking from itching disrupts their rest [38]. Decreased sleep quality can impact a person’s ability to concentrate, make decisions, and participate in activities. If this condition persists, it can ultimately lead to a decline in academic performance [39]. In the Bijagós community, scabies is heavily stigmatized due to concerns about its contagious nature. This stigma is linked to discriminatory attitudes, feelings of rejection, social isolation, and specific behaviors related to seeking treatment. The delay in seeking medical help and the resulting health issues may be exacerbated by the stigma surrounding the condition. Thus, students in the community may feel deeply affected and embarrassed to attend class [40].
Considering the risk factors prevalent in LMICs, both individual and environmental hygiene issues significantly contribute to the overall risk profile. Additionally, most communities in LMICs live below the poverty line, a condition closely linked to inadequate attention to hygiene [41, 42]. Environmental management appears to be a promising intervention to reduce the incidence of scabies. For instance, a boarding school in Indonesia implemented an intensive cleaning campaign over three consecutive weeks, leading to improved hygiene practices among the students. However, such campaigns alone are insufficient; they must be complemented by comprehensive health education on scabies [43]. Lopes et al. [44] conducted educational activities in Nigeria among boarding school students under 11 years old, using a learning module presented by a dermatologist with engaging PowerPoint slides. The results are positive, showing increased knowledge and behavioral changes among the students. Nevertheless, it is crucial for the government to ensure that this educational program is implemented routinely for optimal results, and it should not be prohibitively expensive.
A study in Bangladesh implemented a four-month program to control scabies in an Islamic boarding school. The program include daily monitoring of personal hygiene, weekly health education classes, and monthly meetings between class monitors and scabies control officers. As a result, the school leadership, dormitory caregivers, and all students are fully committed to maintaining personal hygiene and the cleanliness of the dormitory environment [45].
One of the limitations of this study is the quality of the studies. Most included studies were of low or fair quality, raising concerns about the generalizability of the findings. Also, limited access to high-quality journals may have skewed the results toward studies published in less reputable sources. The majority of studies originated from Asia, with only a few from Africa. This limits the generalizability to other LMICs. Therefore, caution should be exercised when using the results of these studies.
This study identified several risk factors with strong significance for the incidence of scabies in children in LMICs. Additionally, this study identified differences in risk factors between students who attended public schools and those in boarding schools or Islamic boarding schools. To effectively prevent and control scabies in children, public health initiatives must consider a comprehensive range of contributing factors. This can be achieved through multisectoral strategies and partnerships that address all the elements involved in scabies transmission.
Conclusion
Gender, age, family economic status, and personal hygiene are risk factors for scabies in schoolchildren in LMICs.
Acknowledgments: We would like to express our gratitude to the Head of Jambi Health Polytechnic who provided material support in the implementation of this study.
Ethical Permissions: Not applicable.
Conflicts of Interests: The authors declared no conflicts of interests.
Authors' Contribution: Supriadi S (First Author), Introduction Writer/Main Researcher/Methodologist/Discussion Writer/Statistical Analyst (40%); Naswir M (Second Author), Assistant Researcher/Discussion Writer (20%); Johari A (Third Author), Introduction Writer/Assistant Researcher/Discussion Writer (20%); Fahri S (Fourth Author), Introduction Writer/Assistant Researcher/Discussion Writer (20%)
Funding/Support: This research received no external funding.
Article Type: Systematic Review |
Subject:
Social Determinants of Health
Received: 2024/07/18 | Accepted: 2024/09/2 | Published: 2024/09/28
Received: 2024/07/18 | Accepted: 2024/09/2 | Published: 2024/09/28
References
1. Bhat SA, Mounsey KE, Liu X, Walton SF. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. [Link] [DOI:10.1186/s13071-017-2320-4]
2. El-Moamly AA. Scabies as a part of the World Health Organization roadmap for neglected tropical diseases 2021-2030: What we know and what we need to do for global control. Trop Med Health. 2021;49(1):64. [Link] [DOI:10.1186/s41182-021-00348-6]
3. WHO. Scabies [Internet]. Geneva: World Health Organization; 2023 [cited 2023, May, 31]. Available from: https://www.who.int/news-room/fact-sheets/detail/scabies. [Link]
4. Engelman D, Steer AC. Control strategies for scabies. Trop Med Infect Dis. 2018;3(3):98. [Link] [DOI:10.3390/tropicalmed3030098]
5. Thean LJ, Engelman D, Kaldor J, Steer AC. Scabies: New opportunities for management and population control. Pediatr Infect Dis J. 2019;38(2):211-3. [Link] [DOI:10.1097/INF.0000000000002211]
6. Karimkhani C, Colombara DV, Drucker AM, Norton SA, Hay R, Engelman D, et al. The global burden of scabies: A cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(12):1247-54. [Link] [DOI:10.1016/S1473-3099(17)30483-8]
7. Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373(24):2371-2. [Link] [DOI:10.1056/NEJMe1511805]
8. Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134(6):1527-34. [Link] [DOI:10.1038/jid.2013.446]
9. Fitzgerald D, Kevitt FA, Reid A. Treatment of close contacts of people with scabies for preventing re‐infestation or spread of infestation in contacts. Cochrane Database Syst Rev. 2012;(7):CD009943. [Link] [DOI:10.1002/14651858.CD009943]
10. Leung AKC, Lam JM, Leong KF. Scabies: A neglected global disease. Curr Pediatr Rev. 2020;16(1):33-42. [Link] [DOI:10.2174/1573396315666190717114131]
11. Yusof MBM, Fitri S, Damopolii Y. A study on knowledge, attitude and practice in preventing transmission of scabies in Pesantren Darul Fatwa, Jatinangor. Althea Med J. 2015;2(1):131-7. [Link] [DOI:10.15850/amj.v2n1.448]
12. Jetly K, Ibrahim FE, Karim IKA, Jeevanathan C, Mokti K, Pang NTP, et al. Risk factors for scabies in school children: A systematic review. Universiti Malaysia Sabah. 2022;17(2):117-25. [Link] [DOI:10.20953/1817-7646-2022-2-117-125]
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [Link] [DOI:10.1136/bmj.n71]
14. Arisandi Y, Anwar C, Salni, Hikmah Purnama D, Novrikasari, Ghiffari A. The dominant factors of scabies incidence in two Islamic boarding school students, South Sumatera, Indonesia. E3S Web Conf. 2018;68(2018):01018. [Link] [DOI:10.1051/e3sconf/20186801018]
15. Maleki Birjandi M, Oroei M, Emadi SN, Peyvandi AA, Kwabena Anang A. Scabies among high school students in Accra, Ghana: Risk factors and health literacy. Iran Red Crescent Med J. 2019;21(8):1-8. [Link] [DOI:10.5812/ircmj.92510]
16. Dagne H, Dessie A, Destaw B, Yallew WW, Gizaw Z. Prevalence and associated factors of scabies among schoolchildren in Dabat district, northwest Ethiopia, 2018. Environ Health Prev Med. 2019;24(1):67. [Link] [DOI:10.1186/s12199-019-0824-6]
17. Ejigu K, Haji Y, Toma A, Tadesse BT. Factors associated with scabies outbreaks in primary schools in Ethiopia: A case-control study. Res Rep Trop Med. 2019;10:119-27. [Link] [DOI:10.2147/RRTM.S214724]
18. Hidayat UA, Hidayat AA, Bahtiar Y. The relationship between the level of knowledge about scabies and the incidence of scabies in Manbaul Ulum students. Jurnal Keperawatan Galuh. 2022;4(2). [Indonesian] [Link] [DOI:10.25157/jkg.v4i2.7817]
19. Kouotou EA, Nansseu JRN, Kouawa MK, Zoung-Kanyi Bissek AC. Prevalence and drivers of human scabies among children and adolescents living and studying in Cameroonian boarding schools. Parasit Vectors. 2016;9(1):400. [Link] [DOI:10.1186/s13071-016-1690-3]
20. Argentina F, Harahap DH, Lusiana E. Riskfactors of scabies in students of Aulia Cendikia Islamic BoardingSchool, Palembang. Jurnal Kedokteran dan Kesehatan: Publikasi Ilmiah Fakultas Kedokteran Universitas Sriwijaya. 2019;6(3):96-100. [Link] [DOI:10.32539/JKK.V6I3.9851]
21. Nanda FD, Murti B, Dharmawan R. Path analysis on factors associated with the risk of scabies among students at Darussalam Islamic Boarding School, Blokagung, Banyuwangi, Indonesia. J Epidemiol Public Health. 2016;1(1):18-26. [Link] [DOI:10.26911/jepublichealth.2016.01.01.03]
22. Rihatmadja R, Miranda E, Wicaksono MM, Widaty S. Why are they hard to treat? A preliminary survey to predict important factors causing persistent scabies among students of religion-affiliated boarding schools in Indonesia. Dermatol Rep. 2019;11(S1):8033. [Link] [DOI:10.4081/dr.2019.8033]
23. Sanei-Dehkordi A, Soleimani-Ahmadi M, Zare M, Jaberhashemi SA. Risk factors associated with scabies infestation among primary schoolchildren in a low socio-economic area in southeast of Iran. BMC Pediatr. 2021;21(1):249. [Link] [DOI:10.1186/s12887-021-02721-0]
24. Yulfi H, Zulkhair M, Yosi A. Scabies infection among boarding school students in Medan, Indonesia: Epidemiology, risk factors, and recommended prevention. Trop Parasitol. 2022;12(1):34-40. [Link] [DOI:10.4103/tp.tp_57_21]
25. Yasmin S, Ullah H, Inayat M, Ullah Khan M, Suleman Dr, Tabassum S, et al. Epidemiological study of scabies among school going children in district Haripur, Pakistan. Arthropods. 2017;6(2):59-66. [Link]
26. Qasim MM. Epidemiology of scabies among primary school children in Quetta. Pak J Med Health Sci. 2015;9(3):903-6. [Link]
27. Ihtiaringtyas S, Mulyaningsih B, Umniyati SR. Risk factors for transmission of scabies in students at the An Nawawi Berjan Islamic Boarding School, Gebang District, Purworejo Regency, Central Java. Balaba: Jurnal Litbang Pengendalian Penyakit Bersumber Binatang Banjarnegara. 2019;15(1):83-90. [Indonesian] [Link] [DOI:10.22435/blb.v15i1.1784]
28. Setiawati E, Zahtamal Z, Putra RM. Analysis of the relationship between risk factors for scabies at the Darel Hikmah Islamic boarding school. SEHATI: Jurnal Kesehatan. 2022;2(2):61-71. [Indonesian] [Link] [DOI:10.52364/sehati.v2i2.29]
29. Normaznah Y, Saniah K, Nazma M, Mak JW, Krishnasamy M, Hakim SL. Seroprevalence of sarcoptes scabiei var canis antibodies among aborigines in peninsular Malaysia. Southeast Asian J Trop Med Public Health. 1996;27(1):53-6. [Link]
30. Nwufoh OC, Sadiq AN, Emikpe BO. The seroprevalence of Sarcoptes scabiei var. canis and its associated risk factors in dogs in Ibadan, Southwest Nigeria. J Immunoassay Immunochem. 2019;40(5):473-84. [Link] [DOI:10.1080/15321819.2019.1631845]
31. Houck E, Olfenbuttel C, Stoskopf M, Kennedy-Stoskopf S. Seroprevalence of sarcoptes scabiei in free-ranging black bears (ursus americanus) in Eastern North Carolina, USA. J Wildl Dis. 2021;57(3):628-31. [Link] [DOI:10.7589/JWD-D-20-00091]
32. Romani L, Whitfeld MJ, Koroivueta J, Kama M, Wand H, Tikoduadua L, et al. The epidemiology of scabies and impetigo in relation to demographic and residential characteristics: Baseline findings from the skin health intervention Fiji trial. Am J Trop Med Hyg. 2017;97(3):845-50. [Link] [DOI:10.4269/ajtmh.16-0753]
33. Romani L, Koroivueta J, Steer AC, Kama M, Kaldor JM, Wand H, et al. Scabies and impetigo prevalence and risk factors in Fiji: A national survey. PLoS Negl Trop Dis. 2015;9(3):e0003452. [Link] [DOI:10.1371/journal.pntd.0003452]
34. Hegab DS, Kato AM, Kabbash IA, Dabish GM. Scabies among primary schoolchildren in Egypt: Sociomedical environmental study in Kafr El-Sheikh administrative area. Clin Cosmet Investig Dermatol. 2015;8:105-11. [Link] [DOI:10.2147/CCID.S78287]
35. Sarma K, Roychoudhury P, Das G, Borthaku SK, Kalita G, Prasad H, et al. Seroprevalence of sarcoptes scabiei var suis infestation in swine population and its effect on haemato-biochemical and oxidative stress indices and its management with special reference to herbal ointmen. Indian J Anim Res. 2019;53(11):1489-96. [Link] [DOI:10.18805/ijar.B-3672]
36. Maharani CS, Harnanti DV, Mappamasing H, Widyantari SS, Ervianty E, Murtiastutik D, et al. Crusted scabies in patients with long-term use of oral corticosteroid with different underlying diseases-case series. Dermatol Rep. 2019;11(s1):183-5. [Link] [DOI:10.4081/dr.2019.8095]
37. Karthikeyan K. Scabies in children. Arch Dis Child Educ Pract Ed. 2007;92(3):ep65-9. [Link] [DOI:10.1136/adc.2005.073825]
38. Glennie M, Gardner K, Dowden M, Currie BJ. Active case detection methods for crusted scabies and leprosy: A systematic review. PLoS Negl Trop Dis. 2021;15(7):e0009577. [Link] [DOI:10.1371/journal.pntd.0009577]
39. Redondo-Bravo L, Fernandez-Martinez B, Gómez-Barroso D, Gherasim A, García-Gómez M, Benito A, et al. Scabies in Spain? A comprehensive epidemiological picture. PLoS One. 2021;16(11):e0258780. [Link] [DOI:10.1371/journal.pone.0258780]
40. Menaldi S, Surya D, The VV. Impact of scabies on Indonesian public boarding school students' quality of life: A mixed-method analysis. J Gen Proced Dermatol Venereol Indones. 2021;5(2):1. [Link] [DOI:10.19100/jdvi.v5i2.264]
41. Merti LGIA, Mutiara H, Fatriyadi Suwandi J, Ayu PR. Relationship scabies with learning achievment on santri boarding school at Bandar Lampung. Medula. 2019;8(2):76-81. [Indonesian] [Link]
42. Jackson A, Heukelbach J, Filho AFDS, Campelo EDB, Feldmeier H. Clinical features and associated morbidity of scabies in a rural community in Alagoas, Brazil. Trop Med Int Health. 2007;12(4):493-502. [Link] [DOI:10.1111/j.1365-3156.2006.01809.x]
43. Jalali R, Khazaei H, Paveh BK, Hayrani Z, Menati L. The effect of sleep quality on students' academic achievement. Adv Med Educ Pract. 2020;11:497-502. [Link] [DOI:10.2147/AMEP.S261525]
44. Lopes MJ, Da Silva ET, Ca J, Gonçalves A, Rodrigues A, Mandjuba C, et al. Perceptions, attitudes and practices towards scabies in communities on the Bijagós Islands, Guinea-Bissau. Trans R Soc Trop Med Hyg. 2020;114(1):49-56. [Link] [DOI:10.1101/574327]
45. Sarkingobir Y, Umar AI, Gidadawa FA, Miya YY. Assessment of food security, living condition, personal hygiene health determinants and relations among Almajiri students in Sokoto metropolis, Nigeria. J Thu Dau Mot Univ. 2023;5(1):63-76. [Link] [DOI:10.37550/tdmu.EJS/2023.01.372]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






