Volume 12, Issue 2 (2024)
Health Educ Health Promot 2024, 12(2): 281-290 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Suharti S, Daryono D, Dewi M, Masyitah D. Incidence of Diabetes Mellitus in Children and Adolescents; A Systematic Review. Health Educ Health Promot 2024; 12 (2) :281-290
URL: http://hehp.modares.ac.ir/article-5-72942-en.html
URL: http://hehp.modares.ac.ir/article-5-72942-en.html
1- Nursing Department, Health Polytechnic of Ministry of Health Jambi, Jambi, Indonesia
Keywords: Diabetes Mellitus [MeSH], Diabetes Mellitus, Type 1 [MeSH], Diabetes Mellitus, Type 2 [MeSH], Child [MeSH], Adolescent [MeSH], Obesity [MeSH]
Full-Text [PDF 794 kb]
(3224 Downloads)
| Abstract (HTML) (1566 Views)
Full-Text: (304 Views)
Introduction
Diabetes mellitus (DM) is a chronic metabolic condition that cannot be cured but can be managed. It may develop due to hereditary factors or environmental influences and lifestyle choices. DM arises from impaired insulin secretion or action, leading to abnormalities in carbohydrate, protein, and lipid metabolism. The reduction in insulin secretion and increased resistance form the basis for categorizing DM into type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM). According to global surveys, a majority of children and adolescents across various age groups are affected by T1DM [1].
While T1DM remains the most commonly observed type among children and adolescents, there has been a notable rise in the occurrence of T2DM within this age demographic [2, 3]. Additionally, other less prevalent types of diabetes have been identified within the community, including monogenic diabetes, medication-induced diabetes, and diabetes triggered by secondary diseases [4]. The diagnostic criteria used for identifying DM and the heightened risk of developing diabetes (prediabetes) in children and adolescents mirror those employed for adults [5]. Nonetheless, the treatment and management of diabetes in youngsters involve unique considerations [6]. These factors include alterations in insulin sensitivity, reliance on medication, and neurological issues associated with the instability of blood sugar levels [7]. The escalating prevalence of childhood obesity correlates directly with the increased incidence and prevalence of T2DM, complicating the differentiation between T2DM and T1DM since more children diagnosed with T1DM are overweight at diagnosis. Additionally, research has indicated the presence of β-cell autoimmunity in children affected by T2DM [8].
Identifying the factors influencing shifts in the epidemiology of DM in children and adolescents is crucial. This age group represents a vital phase for shaping habits and mitigating the risk of diabetes onset from adjustable factors like obesity and sedentary behavior [9]. Studies have highlighted that preventive strategies for T1DM have not achieved maximum success, and the precise risk factors remain unclear [10]. Nevertheless, certain research has unveiled connections between modifiable and non-modifiable factors linked to DM in children and teenagers [11]. Apart from a strong hereditary predisposition to the disease, additional elements such as rapid economic growth leading to dietary shifts, breastfeeding practices, and epigenetic modifications associated with an unfavorable prenatal environment have been recognized [12]. Similar risk factors are prevalent in children and adolescents residing in Asia [13], as well as in the Middle East and North Africa [14], regions witnessing a significant surge in diabetes cases within this age range. Both types of diabetes (T1DM and T2DM), along with an elevated susceptibility to pre-diabetes, are indeed present among children and adolescents [15, 16].
One review encountered during our literature search focused on interventions tailored for adolescents dealing with T2DM. The study emphasized the underuse of pediatric registries, recognizing their role as platforms for sharing data, swiftly generating insights, and guiding decisions to enhance health outcomes [17]. Additionally, Lascar et al. [18] conducted a study summarizing the epidemiology, risk factors, complications, and management of T2D in adolescents. They concluded that there is a necessity to explore lifestyle measures for prevention within family dynamics and improve health education within school programs. Despite the growing attention toward T2D, gaps in knowledge persist regarding its epidemiology due to incomplete enrollment and limited population-based investigations examining the widespread presence of prediabetes and undiagnosed T2DM in adolescents [19]. In adults, obesity is considered a significant contributor to the risk of DM, alongside other key factors like age, race/ethnicity, family medical history, lack of physical activity, and unhealthy dietary habits [20]. Consequently, there's a pressing need for more extensive research focused on understanding and tackling the diabetes and prediabetes epidemic, specifically investigating the primary risk factors affecting children and adolescents.
The present study aimed to systematically review and assess the risk elements linked to diabetes within this younger age demographic.
Information and methods
Protocol
The present systematic review complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [21]. The review included published studies from 2000 to 2023.
Search strategy
We conducted searches across multiple databases, including PubMed, ScienceDirect, Cochrane Library, and Medline. The search terms used for articles were "Diabetes Mellitus," "T1DM," "T2DM," "Child," and "Adolescent," tailored to match the vocabulary of each specific database. These keywords were combined using Boolean operators like "AND" and "OR." Our search was restricted to articles available in English. We further refined the filters to yield more precise and relevant results.
Inclusion and exclusion criteria
This review encompassed all research examining risk factors for DM in children and adolescents. There was no restriction on the search timeframe to ensure a thorough compilation of relevant studies. However, studies published in conference proceedings, reviews, meta-analyses, commentaries, book chapters, reports, case studies, and letters to the editor were excluded from consideration.
Selection process
Two authors independently and separately screened the titles or abstracts of the studies. In cases of conflicting opinions between the two screening authors, the first author made the final decision. This rapid screening involved assessing the titles, objectives, and conclusions of the pertinent studies. Further investigation into specific studies was pursued by delving into the main text to gather additional information as needed.
Study quality
The evaluation of article quality utilized the National Institutes of Health (NIH) tool designed for controlled intervention studies. This assessment tool comprises 14 statements gauging the comprehensiveness of reports derived from the reviewed studies. The assessment criteria are categorized based on the number of affirmative responses (YES). Results were categorized as follows: a score of <30% labeled as "poor," 30-70% as "fair," and a score exceeding 70% considered as "good" according to the provided guidelines.
Risk of bias
The Risk of Bias in Non-Randomized Studies-Exposure Studies (ROBINS-E) tool was used to evaluate the types of bias in each study [22]. It consists of seven domains that assess the internal and external validity of the study. The assessment results of these domains are categorized as low, some concerns, high, and very high. The risk of bias assessment results were reviewed and approved by all authors, considering the opinions of external reviewers.
Data extraction and synthesis
To facilitate comprehension of the eligible studies' contents, crucial information was summarized (Table 1). Two authors collaborated in this extraction process. In cases where there were differing opinions regarding the extracted data, they were reconciled through consensus. The extraction criteria included the first author and publication year, study aim, observational design, participants, modifiable and non-modifiable factors, as well as key findings.
Table 1. Characteristics of the included studies
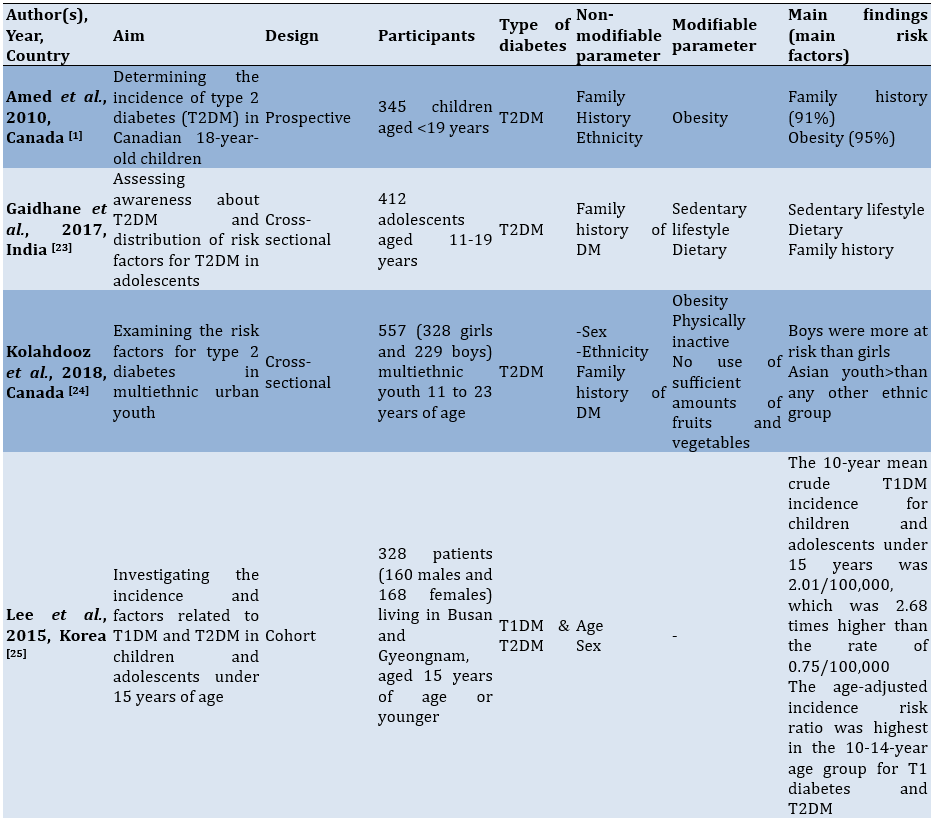
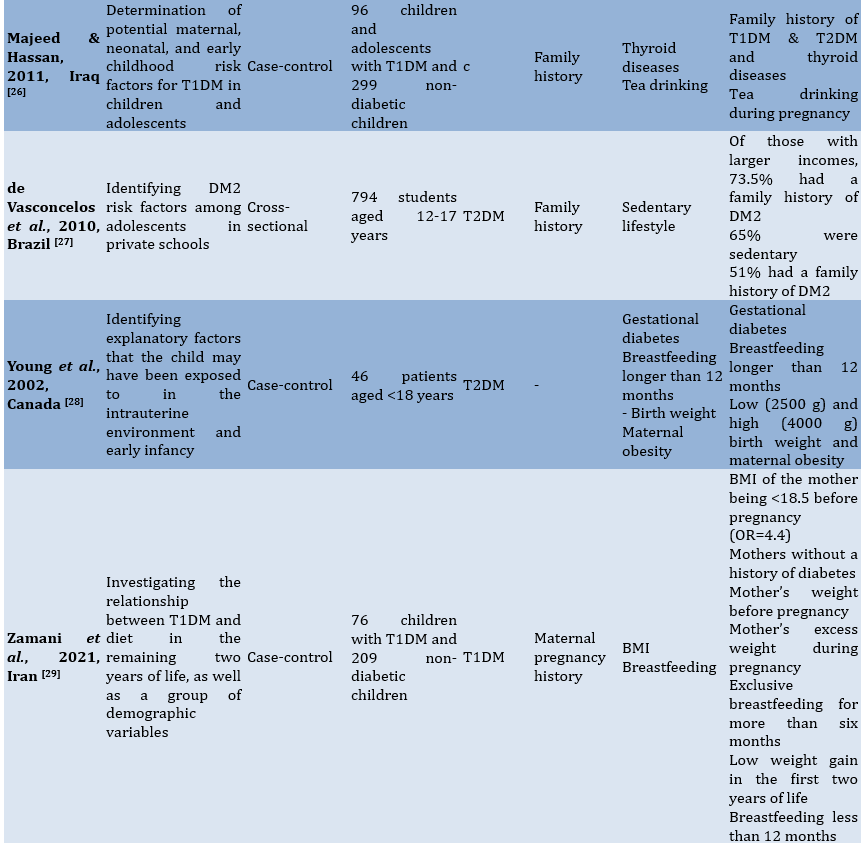
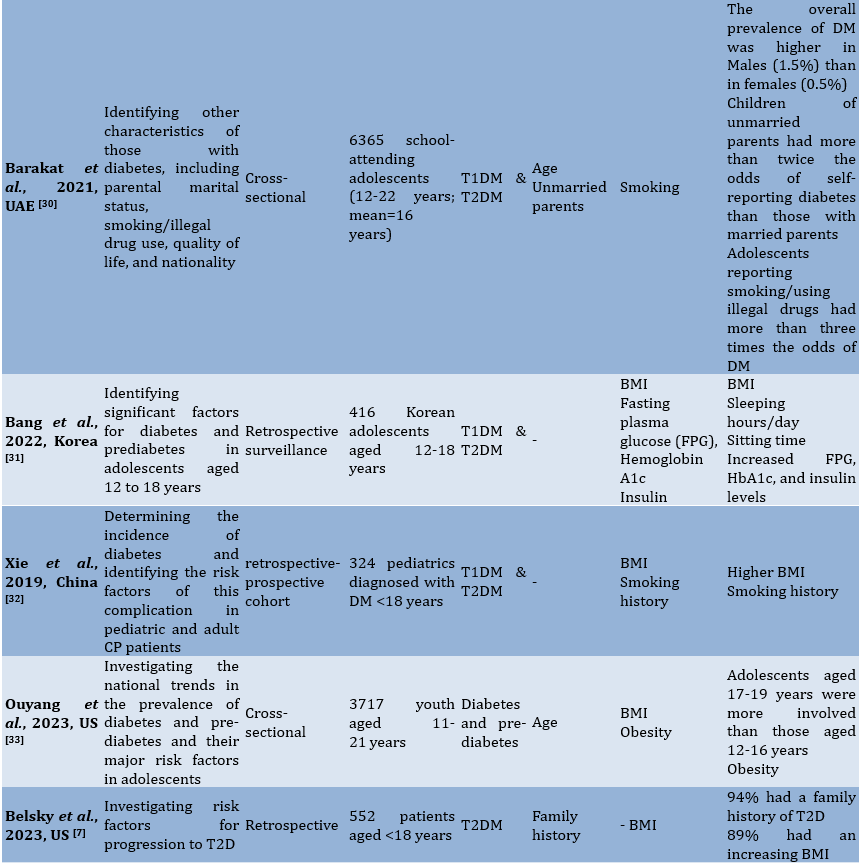
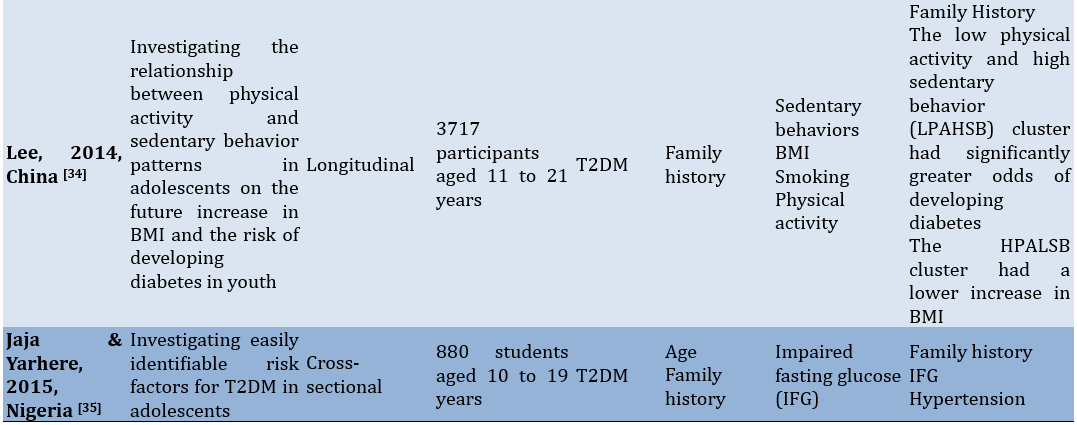
Findings
The initial database search retrieved 9949 articles. After removing 5430 duplicates and ineligible articles which were not relevant to the review's theme, 4519 articles remained for screening. During the eligibility assessment, 54 studies were evaluated, and 39 articles were excluded for various reasons. Ultimately, only 15 studies met the criteria and proceeded to the next stage of extraction and analysis (Figure 1).
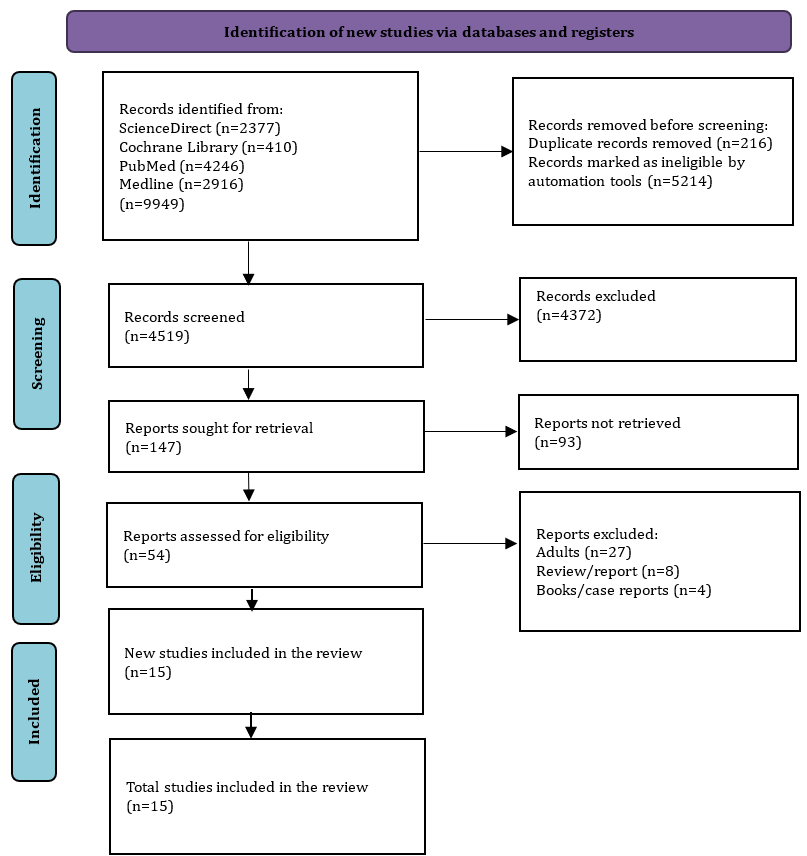
Figure 1. PRISMA flow diagram for literature search
Characteristics of the included studies
The 15 studies reviewed in this analysis came from several countries, including Canada (n=3), Korea (n=2), China (n=2), the US (n=2), and one each from India, Iraq, Brazil, Iran, the UAE, and Nigeria. The study designs included cross-sectional, prospective, cohort, case-control, retrospective, and longitudinal studies. The total number of participants involved in these studies amounted to 19235 children and adolescents.
Quality assessment of the included studies
The study quality assessment using the NIH tool for observational cohort and cross-sectional studies is summarized in Table 2, including the assessment process and summary of the results for each study.
Table 2. Summary of study quality assessment
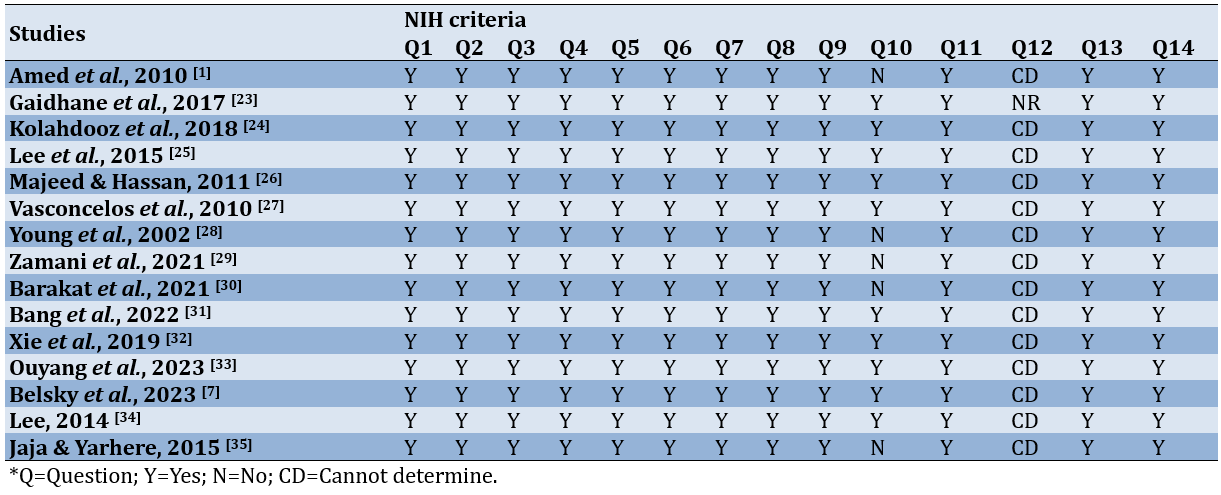
According to the findings from the study quality assessment, the majority of the studies fell within the good quality category. This classification was derived from scores surpassing 70% of the total NIH criteria designated for observational cohort and cross-sectional studies.
Assessment of risk of bias from included studies
The outcomes of the risk of bias assessment for the included studies were showcased through a traffic-light plot (Figure 2). During the assessment process, all authors unanimously concurred with the assessment outcomes without significant debate. External reviewers provided valuable feedback, particularly concerning domain five, which focuses on Missing Data for certain studies.
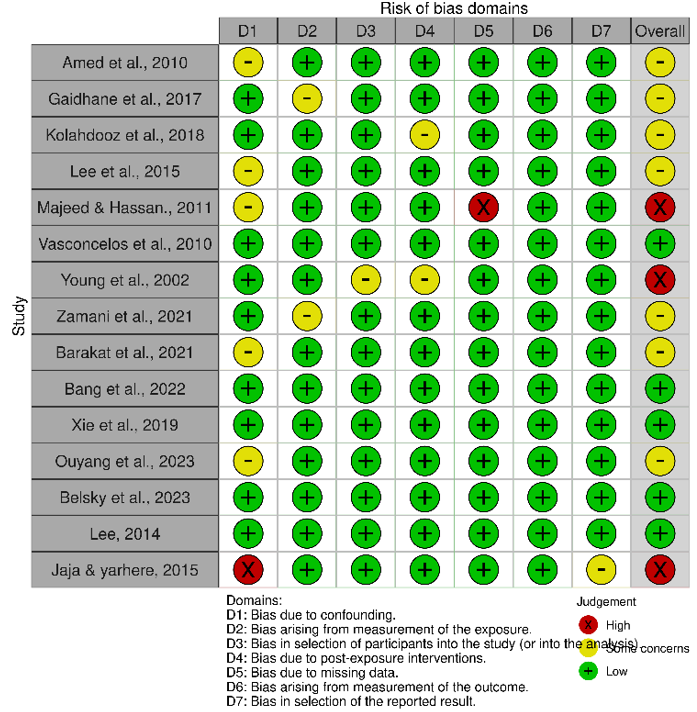
Figure 2. Summary of the risk of bias of the included studies.
The traffic-light plot illustrates 3 studies classified under the high risk of bias category, 7 studies under some concern category, and 5 studies under the low risk of bias category. To ensure the thoroughness of this review, we included three studies despite their placement within the high risk of bias category.
Type of diabetes mellitus targeted by each included study
The focus of most of the studies was on T2DM, with 8 studies specifically dedicated to T2DM, while only 2 studies concentrated on T1DM. Additionally, the remaining 5 studies addressed both types of DM collectively.
Non-modifiable risk factors associated with diabetes mellitus
Among the 15 studies included, the family history of DM emerged as the most extensively discussed and main risk factor [1, 7, 23-27, 34, 35]. Ethnicity was another notable risk factor elaborated in multiple studies (n=4) [1, 7, 24, 33]. Gender was also identified as a risk factor associated with the incidence of DM; statistical analysis results indicated boys>girls in T2DM [24] and male[25]. Age was linked to DM across four studies [24, 25, 30, 33, 35]. Additionally, maternal pregnancy and unmarried parents were found to contribute to the incidence of DM in children, as indicated by two studies [29, 30].
Modifiable risk factors associated with diabetes mellitus
Modifiable risk factors were identified from the 15 studies included in this review. These risk factors included obesity (BMI) as the most dominant risk factor discussed in eight studies [1, 7, 24, 29, 31-34], sedentary behavior [23, 27, 34], physical activity [24, 34], smoking history [30, 32, 34], diet [23, 24, 26, 28, 29], and other conditions, including thyroid disease, gestational diabetes, and hypertension [26, 28, 35].
Discussion
This systematic review provided a comprehensive synthesis of the factors associated with DM in children and adolescents presenting with new-onset T1DM and T2DM. We discovered that obesity emerged as a predominant and frequent risk factor across the studies examined. Its significance surpassed other risk factors in terms of percentage, p-value, and OR. This consistent pattern underscores obesity as a major issue demanding focused attention in the future concerning children and adolescents. Additionally, the statistical significance of sedentary behavior as a risk factor indicates a substantial relationship. This is supported by prior research, which established a link between overweight and obesity with increased levels of sedentary behaviors (RRR=1.33; 1.27-1.39 and RRR=1.73; 1.63-1.84) [36].
Understanding the risk elements linked to diabetes in children and teenagers will aid in devising tailored strategies to prevent the onset of such risk factors or manage those already present, especially in younger populations [37]. The rise in diabetes prevalence among this demographic can amplify the occurrence of additional health issues or complications associated with diabetes, including high blood pressure, abnormal lipid levels, retinopathy, and cardiovascular ailments. Furthermore, the emergence and progression of diabetic complications during the early stages of life could potentially present a significant future public health issue [38].
In terms of non-modifiable risk factors, family history emerges as a potent predictor influencing the incidence of DM in children and adolescents, displaying strong significance in most of the studies reviewed. Individuals with first-degree relatives affected by T2DM face a notably heightened risk, being two to six times more prone to developing the disease than those without a family history of diabetes [39]. This strong association underscores the genetic component's pivotal role, indicating that individuals from parents with diabetes carry a five to tenfold higher likelihood of developing the condition compared to the general population [40]. Furthermore, other research indicates that this predisposition elevates the likelihood of obesity, decreased HDL levels, and heightened blood pressure among adolescents of Asian Indian descent [41]. Children and teenagers in Mexico with a family history of diabetes face higher occurrences of hypertension, obesity, elevated insulin and triglyceride levels, along with an increased insulin resistance index, in contrast to those from non-diabetic families [42].
Non-modifiable risk factors also contribute to diabetes in children and adolescents. Lee [25] identify a substantial increase in the incidence rate ratio for both T1DM and T2DM within the age group of 10 to 14 years, ranging from 1.56 to 2.87. Furthermore, another study highlights a surge in diabetes incidence among individuals aged 17 to 19 years, showing statistically significant results [33]. Consistent with Lee’s findings, Jaja & Yarhere [35] observe a rise in diabetes incidence within a similar age range of 10 to 12.9 years, which serve as the reference group in their study. A Korean study reinforces these patterns, indicating a 6.39 times higher prevalence of diabetes among adolescents aged 10 to 14 years and a 5.34 times higher prevalence among those aged 15 to 19 years. The increasing occurrence of diabetes in Korean teenagers is noticeable at younger ages, presenting challenges in comprehending and managing their health, particularly in handling diabetes-related risks, which can be arduous. Hence, the support and guidance provided by parents and school educators are vital in assisting these adolescents [31]. However, in the UAE, age does not seem to be a significant predictor for the incidence of diabetes in adolescents, as noted in a study by Barakat et al. [30]. Likewise, in a study in United States, the statistical results do not show significant concerning age. Regarding ethnicity, findings from different studies present varying outcomes. For Asian adolescents exhibit a higher incidence compared to other ethnic groups [24], while another study pointed out that Mexican-American ethnicity has a higher prevalence of diabetes [33]. As a result, the authors have chosen to temporarily set aside ethnicity as a risk factor for diabetes incidence among children and adolescents. This emphasizes the importance of conducting comprehensive studies involving larger population samples with diverse ethnic backgrounds analyzed collectively or within a cohort framework.
Within modifiable risk factors, several elements exhibit significant importance, such as obesity, sedentary lifestyles, and physical activity. Obesity is a well-known crucial risk factor in the development of diabetes mellitus in children and adolescents. Children grappling with obesity face a significantly increased risk of encountering various health complications, both physical and psychological. The root of obesity often traces back to the concept of early adiposity rebound, signifying the phase when a child’s BMI starts to rise after reaching its lowest fat level, typically occurring around the age of 5 or 6. Individuals experiencing adiposity rebound as early as 3 years old often observe a consistent increase in their BMI from early childhood through adolescence and into adulthood [43]. Understanding the link between obesity and T2DM involves complex physiological and cellular mechanisms, encompassing changes induced by excess fat in beta-cell function, adipose tissue biology, and insulin resistance across various organs. These factors can often improve and potentially normalize with significant weight loss.
In India, a sedentary lifestyle is more prevalent among adolescents aged 15-19 years compared to younger age groups [23]. Vasconcelos et al. highlight that the most significant risk factors are overweight and sedentary behavior [27]. Research clearly indicates that both sedentary behaviors and physical activity independently contribute to the development of T2DM among adults [44, 45]. However, this specific study concentrated on confirming the impact of sedentary behaviors [46] among adolescents without delving into the effects of physical activity. Several reasons could elucidate this observation. Initially, prior suggestions propose that the relationship between physical activity and type 2 diabetes might be influenced by body fat [47]. Consequently, the 14-year follow-up solely monitor body fat management through physical activity, necessitating a longer duration to comprehensively grasp the correlation between body fat and T2DM. Secondly, a previous study emphasizes on a stronger link between blood glucose levels and cardiorespiratory fitness in comparison to energy expenditure [48], indicating that the heart and lungs’ ability to function effectively could act as the mediator between physical activity and T2DM. Thirdly, the most prevalent sedentary behavior in the studies examine TV viewing, which is associated with unhealthy dietary patterns [5], potentially elucidating the connection between sedentary behaviors and T2DM. Hence, it is plausible that a lack of physical activity contributes to changes in BMI.
This review may not comprehensively encapsulate studies published in reputable journals. It also encompassed three studies categorized as High Risk of Bias; hence, caution should be exercised when utilizing the results of this review. The findings advocate for a focus on diabetes prevention in children and adolescents by highlighting weight management, reducing sedentary behaviors, and promoting physical activity. Additionally, attention to other factors such as smoking, dietary patterns, and increasing fruit intake is advised [49, 50].
Conclusion
There are no significant differences in risk factors for T1DM and T2DM.
Acknowledgments: Nothing declared by the authors.
Ethical Permissions: Not applicable.
Conflicts of Interests: The authors reported no conflicts of interests.
Authors’ Contribution: Suharti S (First Author), Introduction Writer/Methodologist/Main Researcher (40%); Daryono D (Second Author), Assistant Researcher/Discussion Writer (20%); Dewi M (Third Author), Assistant Researcher/Statistical Analyst (20%); Masyitah D (Fourth Author), Methodologist/Assistant Researcher (20%)
Funding/Support: No funding was received.
Diabetes mellitus (DM) is a chronic metabolic condition that cannot be cured but can be managed. It may develop due to hereditary factors or environmental influences and lifestyle choices. DM arises from impaired insulin secretion or action, leading to abnormalities in carbohydrate, protein, and lipid metabolism. The reduction in insulin secretion and increased resistance form the basis for categorizing DM into type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM). According to global surveys, a majority of children and adolescents across various age groups are affected by T1DM [1].
While T1DM remains the most commonly observed type among children and adolescents, there has been a notable rise in the occurrence of T2DM within this age demographic [2, 3]. Additionally, other less prevalent types of diabetes have been identified within the community, including monogenic diabetes, medication-induced diabetes, and diabetes triggered by secondary diseases [4]. The diagnostic criteria used for identifying DM and the heightened risk of developing diabetes (prediabetes) in children and adolescents mirror those employed for adults [5]. Nonetheless, the treatment and management of diabetes in youngsters involve unique considerations [6]. These factors include alterations in insulin sensitivity, reliance on medication, and neurological issues associated with the instability of blood sugar levels [7]. The escalating prevalence of childhood obesity correlates directly with the increased incidence and prevalence of T2DM, complicating the differentiation between T2DM and T1DM since more children diagnosed with T1DM are overweight at diagnosis. Additionally, research has indicated the presence of β-cell autoimmunity in children affected by T2DM [8].
Identifying the factors influencing shifts in the epidemiology of DM in children and adolescents is crucial. This age group represents a vital phase for shaping habits and mitigating the risk of diabetes onset from adjustable factors like obesity and sedentary behavior [9]. Studies have highlighted that preventive strategies for T1DM have not achieved maximum success, and the precise risk factors remain unclear [10]. Nevertheless, certain research has unveiled connections between modifiable and non-modifiable factors linked to DM in children and teenagers [11]. Apart from a strong hereditary predisposition to the disease, additional elements such as rapid economic growth leading to dietary shifts, breastfeeding practices, and epigenetic modifications associated with an unfavorable prenatal environment have been recognized [12]. Similar risk factors are prevalent in children and adolescents residing in Asia [13], as well as in the Middle East and North Africa [14], regions witnessing a significant surge in diabetes cases within this age range. Both types of diabetes (T1DM and T2DM), along with an elevated susceptibility to pre-diabetes, are indeed present among children and adolescents [15, 16].
One review encountered during our literature search focused on interventions tailored for adolescents dealing with T2DM. The study emphasized the underuse of pediatric registries, recognizing their role as platforms for sharing data, swiftly generating insights, and guiding decisions to enhance health outcomes [17]. Additionally, Lascar et al. [18] conducted a study summarizing the epidemiology, risk factors, complications, and management of T2D in adolescents. They concluded that there is a necessity to explore lifestyle measures for prevention within family dynamics and improve health education within school programs. Despite the growing attention toward T2D, gaps in knowledge persist regarding its epidemiology due to incomplete enrollment and limited population-based investigations examining the widespread presence of prediabetes and undiagnosed T2DM in adolescents [19]. In adults, obesity is considered a significant contributor to the risk of DM, alongside other key factors like age, race/ethnicity, family medical history, lack of physical activity, and unhealthy dietary habits [20]. Consequently, there's a pressing need for more extensive research focused on understanding and tackling the diabetes and prediabetes epidemic, specifically investigating the primary risk factors affecting children and adolescents.
The present study aimed to systematically review and assess the risk elements linked to diabetes within this younger age demographic.
Information and methods
Protocol
The present systematic review complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [21]. The review included published studies from 2000 to 2023.
Search strategy
We conducted searches across multiple databases, including PubMed, ScienceDirect, Cochrane Library, and Medline. The search terms used for articles were "Diabetes Mellitus," "T1DM," "T2DM," "Child," and "Adolescent," tailored to match the vocabulary of each specific database. These keywords were combined using Boolean operators like "AND" and "OR." Our search was restricted to articles available in English. We further refined the filters to yield more precise and relevant results.
Inclusion and exclusion criteria
This review encompassed all research examining risk factors for DM in children and adolescents. There was no restriction on the search timeframe to ensure a thorough compilation of relevant studies. However, studies published in conference proceedings, reviews, meta-analyses, commentaries, book chapters, reports, case studies, and letters to the editor were excluded from consideration.
Selection process
Two authors independently and separately screened the titles or abstracts of the studies. In cases of conflicting opinions between the two screening authors, the first author made the final decision. This rapid screening involved assessing the titles, objectives, and conclusions of the pertinent studies. Further investigation into specific studies was pursued by delving into the main text to gather additional information as needed.
Study quality
The evaluation of article quality utilized the National Institutes of Health (NIH) tool designed for controlled intervention studies. This assessment tool comprises 14 statements gauging the comprehensiveness of reports derived from the reviewed studies. The assessment criteria are categorized based on the number of affirmative responses (YES). Results were categorized as follows: a score of <30% labeled as "poor," 30-70% as "fair," and a score exceeding 70% considered as "good" according to the provided guidelines.
Risk of bias
The Risk of Bias in Non-Randomized Studies-Exposure Studies (ROBINS-E) tool was used to evaluate the types of bias in each study [22]. It consists of seven domains that assess the internal and external validity of the study. The assessment results of these domains are categorized as low, some concerns, high, and very high. The risk of bias assessment results were reviewed and approved by all authors, considering the opinions of external reviewers.
Data extraction and synthesis
To facilitate comprehension of the eligible studies' contents, crucial information was summarized (Table 1). Two authors collaborated in this extraction process. In cases where there were differing opinions regarding the extracted data, they were reconciled through consensus. The extraction criteria included the first author and publication year, study aim, observational design, participants, modifiable and non-modifiable factors, as well as key findings.
Table 1. Characteristics of the included studies




Findings
The initial database search retrieved 9949 articles. After removing 5430 duplicates and ineligible articles which were not relevant to the review's theme, 4519 articles remained for screening. During the eligibility assessment, 54 studies were evaluated, and 39 articles were excluded for various reasons. Ultimately, only 15 studies met the criteria and proceeded to the next stage of extraction and analysis (Figure 1).

Figure 1. PRISMA flow diagram for literature search
Characteristics of the included studies
The 15 studies reviewed in this analysis came from several countries, including Canada (n=3), Korea (n=2), China (n=2), the US (n=2), and one each from India, Iraq, Brazil, Iran, the UAE, and Nigeria. The study designs included cross-sectional, prospective, cohort, case-control, retrospective, and longitudinal studies. The total number of participants involved in these studies amounted to 19235 children and adolescents.
Quality assessment of the included studies
The study quality assessment using the NIH tool for observational cohort and cross-sectional studies is summarized in Table 2, including the assessment process and summary of the results for each study.
Table 2. Summary of study quality assessment

According to the findings from the study quality assessment, the majority of the studies fell within the good quality category. This classification was derived from scores surpassing 70% of the total NIH criteria designated for observational cohort and cross-sectional studies.
Assessment of risk of bias from included studies
The outcomes of the risk of bias assessment for the included studies were showcased through a traffic-light plot (Figure 2). During the assessment process, all authors unanimously concurred with the assessment outcomes without significant debate. External reviewers provided valuable feedback, particularly concerning domain five, which focuses on Missing Data for certain studies.

Figure 2. Summary of the risk of bias of the included studies.
The traffic-light plot illustrates 3 studies classified under the high risk of bias category, 7 studies under some concern category, and 5 studies under the low risk of bias category. To ensure the thoroughness of this review, we included three studies despite their placement within the high risk of bias category.
Type of diabetes mellitus targeted by each included study
The focus of most of the studies was on T2DM, with 8 studies specifically dedicated to T2DM, while only 2 studies concentrated on T1DM. Additionally, the remaining 5 studies addressed both types of DM collectively.
Non-modifiable risk factors associated with diabetes mellitus
Among the 15 studies included, the family history of DM emerged as the most extensively discussed and main risk factor [1, 7, 23-27, 34, 35]. Ethnicity was another notable risk factor elaborated in multiple studies (n=4) [1, 7, 24, 33]. Gender was also identified as a risk factor associated with the incidence of DM; statistical analysis results indicated boys>girls in T2DM [24] and male
Modifiable risk factors associated with diabetes mellitus
Modifiable risk factors were identified from the 15 studies included in this review. These risk factors included obesity (BMI) as the most dominant risk factor discussed in eight studies [1, 7, 24, 29, 31-34], sedentary behavior [23, 27, 34], physical activity [24, 34], smoking history [30, 32, 34], diet [23, 24, 26, 28, 29], and other conditions, including thyroid disease, gestational diabetes, and hypertension [26, 28, 35].
Discussion
This systematic review provided a comprehensive synthesis of the factors associated with DM in children and adolescents presenting with new-onset T1DM and T2DM. We discovered that obesity emerged as a predominant and frequent risk factor across the studies examined. Its significance surpassed other risk factors in terms of percentage, p-value, and OR. This consistent pattern underscores obesity as a major issue demanding focused attention in the future concerning children and adolescents. Additionally, the statistical significance of sedentary behavior as a risk factor indicates a substantial relationship. This is supported by prior research, which established a link between overweight and obesity with increased levels of sedentary behaviors (RRR=1.33; 1.27-1.39 and RRR=1.73; 1.63-1.84) [36].
Understanding the risk elements linked to diabetes in children and teenagers will aid in devising tailored strategies to prevent the onset of such risk factors or manage those already present, especially in younger populations [37]. The rise in diabetes prevalence among this demographic can amplify the occurrence of additional health issues or complications associated with diabetes, including high blood pressure, abnormal lipid levels, retinopathy, and cardiovascular ailments. Furthermore, the emergence and progression of diabetic complications during the early stages of life could potentially present a significant future public health issue [38].
In terms of non-modifiable risk factors, family history emerges as a potent predictor influencing the incidence of DM in children and adolescents, displaying strong significance in most of the studies reviewed. Individuals with first-degree relatives affected by T2DM face a notably heightened risk, being two to six times more prone to developing the disease than those without a family history of diabetes [39]. This strong association underscores the genetic component's pivotal role, indicating that individuals from parents with diabetes carry a five to tenfold higher likelihood of developing the condition compared to the general population [40]. Furthermore, other research indicates that this predisposition elevates the likelihood of obesity, decreased HDL levels, and heightened blood pressure among adolescents of Asian Indian descent [41]. Children and teenagers in Mexico with a family history of diabetes face higher occurrences of hypertension, obesity, elevated insulin and triglyceride levels, along with an increased insulin resistance index, in contrast to those from non-diabetic families [42].
Non-modifiable risk factors also contribute to diabetes in children and adolescents. Lee [25] identify a substantial increase in the incidence rate ratio for both T1DM and T2DM within the age group of 10 to 14 years, ranging from 1.56 to 2.87. Furthermore, another study highlights a surge in diabetes incidence among individuals aged 17 to 19 years, showing statistically significant results [33]. Consistent with Lee’s findings, Jaja & Yarhere [35] observe a rise in diabetes incidence within a similar age range of 10 to 12.9 years, which serve as the reference group in their study. A Korean study reinforces these patterns, indicating a 6.39 times higher prevalence of diabetes among adolescents aged 10 to 14 years and a 5.34 times higher prevalence among those aged 15 to 19 years. The increasing occurrence of diabetes in Korean teenagers is noticeable at younger ages, presenting challenges in comprehending and managing their health, particularly in handling diabetes-related risks, which can be arduous. Hence, the support and guidance provided by parents and school educators are vital in assisting these adolescents [31]. However, in the UAE, age does not seem to be a significant predictor for the incidence of diabetes in adolescents, as noted in a study by Barakat et al. [30]. Likewise, in a study in United States, the statistical results do not show significant concerning age. Regarding ethnicity, findings from different studies present varying outcomes. For Asian adolescents exhibit a higher incidence compared to other ethnic groups [24], while another study pointed out that Mexican-American ethnicity has a higher prevalence of diabetes [33]. As a result, the authors have chosen to temporarily set aside ethnicity as a risk factor for diabetes incidence among children and adolescents. This emphasizes the importance of conducting comprehensive studies involving larger population samples with diverse ethnic backgrounds analyzed collectively or within a cohort framework.
Within modifiable risk factors, several elements exhibit significant importance, such as obesity, sedentary lifestyles, and physical activity. Obesity is a well-known crucial risk factor in the development of diabetes mellitus in children and adolescents. Children grappling with obesity face a significantly increased risk of encountering various health complications, both physical and psychological. The root of obesity often traces back to the concept of early adiposity rebound, signifying the phase when a child’s BMI starts to rise after reaching its lowest fat level, typically occurring around the age of 5 or 6. Individuals experiencing adiposity rebound as early as 3 years old often observe a consistent increase in their BMI from early childhood through adolescence and into adulthood [43]. Understanding the link between obesity and T2DM involves complex physiological and cellular mechanisms, encompassing changes induced by excess fat in beta-cell function, adipose tissue biology, and insulin resistance across various organs. These factors can often improve and potentially normalize with significant weight loss.
In India, a sedentary lifestyle is more prevalent among adolescents aged 15-19 years compared to younger age groups [23]. Vasconcelos et al. highlight that the most significant risk factors are overweight and sedentary behavior [27]. Research clearly indicates that both sedentary behaviors and physical activity independently contribute to the development of T2DM among adults [44, 45]. However, this specific study concentrated on confirming the impact of sedentary behaviors [46] among adolescents without delving into the effects of physical activity. Several reasons could elucidate this observation. Initially, prior suggestions propose that the relationship between physical activity and type 2 diabetes might be influenced by body fat [47]. Consequently, the 14-year follow-up solely monitor body fat management through physical activity, necessitating a longer duration to comprehensively grasp the correlation between body fat and T2DM. Secondly, a previous study emphasizes on a stronger link between blood glucose levels and cardiorespiratory fitness in comparison to energy expenditure [48], indicating that the heart and lungs’ ability to function effectively could act as the mediator between physical activity and T2DM. Thirdly, the most prevalent sedentary behavior in the studies examine TV viewing, which is associated with unhealthy dietary patterns [5], potentially elucidating the connection between sedentary behaviors and T2DM. Hence, it is plausible that a lack of physical activity contributes to changes in BMI.
This review may not comprehensively encapsulate studies published in reputable journals. It also encompassed three studies categorized as High Risk of Bias; hence, caution should be exercised when utilizing the results of this review. The findings advocate for a focus on diabetes prevention in children and adolescents by highlighting weight management, reducing sedentary behaviors, and promoting physical activity. Additionally, attention to other factors such as smoking, dietary patterns, and increasing fruit intake is advised [49, 50].
Conclusion
There are no significant differences in risk factors for T1DM and T2DM.
Acknowledgments: Nothing declared by the authors.
Ethical Permissions: Not applicable.
Conflicts of Interests: The authors reported no conflicts of interests.
Authors’ Contribution: Suharti S (First Author), Introduction Writer/Methodologist/Main Researcher (40%); Daryono D (Second Author), Assistant Researcher/Discussion Writer (20%); Dewi M (Third Author), Assistant Researcher/Statistical Analyst (20%); Masyitah D (Fourth Author), Methodologist/Assistant Researcher (20%)
Funding/Support: No funding was received.
Article Type: Systematic Review |
Subject:
Social Determinants of Health
Received: 2023/12/14 | Accepted: 2024/04/5 | Published: 2024/06/21
Received: 2023/12/14 | Accepted: 2024/04/5 | Published: 2024/06/21
References
1. Amed S, Dean HJ, Panagiotopoulos C, Sellers EAC, Hadjiyannakis S, Laubscher TA, et al. Type 2 diabetes, medication-induced diabetes, and monogenic diabetes in Canadian children: A prospective national surveillance study. Diabetes Care. 2010;33(4):786-91. [Link] [DOI:10.2337/dc09-1013]
2. Imperatore G, Mayer-Davis EJ, Orchard TJ, Zhong VW. Prevalence and incidence of type 1 diabetes among children and adults in the United States and comparison with non-US countries. In: Diabetes in America. 3rd ed. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases; 2021. [Link]
3. Lawrence JM, Standiford DA, Loots B, Klingensmith GJ, Williams DE, Ruggiero A, et al. Prevalence and correlates of depressed mood among youth with diabetes: The search for diabetes in youth study. Pediatrics. 2006;117(4):1348-58. [Link] [DOI:10.1542/peds.2005-1398]
4. Lora ALM, Espíndola ME, Paz MB, Díaz JMM, Klünder MK. Diabetes in children and adolescents. In: Rodriguez-Saldana J, editor. The diabetes textbook: Clinical principles, patient management and public health issues. Berlin: Springer; 2023. p. 1063-94. [Link] [DOI:10.1007/978-3-031-25519-9_64]
5. Diaz-Valencia PA, Bougnères P, Valleron AJ. Global epidemiology of type 1 diabetes in young adults and adults: A systematic review. BMC Public Health. 2015;15:255. [Link] [DOI:10.1186/s12889-015-1591-y]
6. American Diabetes Association. 13. Children and adolescents: Standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S180-99. [Link] [DOI:10.2337/dc21-S013]
7. Belsky N, Tamaroff J, Shoemaker AH. Risk factors for progression to type 2 diabetes in a pediatric prediabetes clinic population. J Endocr Soc. 2023;7(11):bvad118. [Link] [DOI:10.1210/jendso/bvad118]
8. Bloomgarden ZT. Type 2 diabetes in the young: The evolving epidemic. Diabetes Care. 2004;27(4):998-1010. [Link] [DOI:10.2337/diacare.27.4.998]
9. Boles A, Kandimalla R, Reddy PH. Dynamics of diabetes and obesity: Epidemiological perspective. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1026-36. [Link] [DOI:10.1016/j.bbadis.2017.01.016]
10. Michels A, Zhang L, Khadra A, Kushner JA, Redondo MJ, Pietropaolo M. Prediction and prevention of type 1 diabetes: Update on success of prediction and struggles at prevention. Pediatr Diabetes. 2015;16(7):465-84. [Link] [DOI:10.1111/pedi.12299]
11. Gbadamosi MA, Tlou B. Modifiable risk factors associated with non-communicable diseases among adult outpatients in Manzini, Swaziland: A cross-sectional study. BMC Public Health. 2020;20(1):665. [Link] [DOI:10.1186/s12889-020-08816-0]
12. Berti C, Agostoni C, Davanzo R, Hyppönen E, Isolauri E, Meltzer HM, et al. Early-life nutritional exposures and lifelong health: Immediate and long-lasting impacts of probiotics, vitamin D, and breastfeeding. Nutr Rev. 2017;75(2):83-97. [Link] [DOI:10.1093/nutrit/nuw056]
13. Phan DH, Do VV, Khuong LQ, Nguyen HT, Minh HV. Prevalence of diabetes and prediabetes among children aged 11-14 years old in Vietnam. J Diabetes Res. 2020;2020:7573491. [Link] [DOI:10.1155/2020/7573491]
14. El-Kebbi IM, Bidikian NH, Hneiny L, Nasrallah MP. Epidemiology of type 2 diabetes in the Middle East and North Africa: Challenges and call for action. World J Diabetes. 2021;12(9):1401-25. [Link] [DOI:10.4239/wjd.v12.i9.1401]
15. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [Link] [DOI:10.1016/j.diabres.2021.109119]
16. Dutil C, Chaput JP. Inadequate sleep as a contributor to type 2 diabetes in children and adolescents. Nutr Diabetes. 2017;7(5):e266. [Link] [DOI:10.1038/nutd.2017.19]
17. Hotu S, Carter B, Watson PD, Cutfield WS, Cundy T. Increasing prevalence of type 2 diabetes in adolescents. J Paediatr Child Health. 2004;40(4):201-4. [Link] [DOI:10.1111/j.1440-1754.2004.00337.x]
18. Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6(1):69-80. [Link] [DOI:10.1016/S2213-8587(17)30186-9]
19. Giwa AM, Ahmed R, Omidian Z, Majety N, Karakus KE, Omer SM, et al. Current understandings of the pathogenesis of type 1 diabetes: Genetics to environment. World J Diabetes. 2020;11(1):13-25. [Link] [DOI:10.4239/wjd.v11.i1.13]
20. Börnhorst C, Russo P, Veidebaum T, Tornaritis M, Molnár D, Lissner L, et al. The role of lifestyle and non-modifiable risk factors in the development of metabolic disturbances from childhood to adolescence. Int J Obes. 2020;44(11):2236-45. [Link] [DOI:10.1038/s41366-020-00671-8]
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [Link] [DOI:10.1016/j.ijsu.2021.105906]
22. Higgins J, Morgan R, Rooney A, Taylor K, Thayer K, Silva R, et al. Risk of bias in non-randomized studies-of exposure (ROBINS-E): Launch version. Health Environ Res Online. 2022. [Link]
23. Gaidhane S, Mittal W, Khatib N, Zahiruddin QS, Muntode PA, Gaidhane A. Risk factor of type 2 diabetes mellitus among adolescents from rural area of India. J Family Med Prim Care. 2017;6(3):600-4. [Link] [DOI:10.4103/2249-4863.222025]
24. Kolahdooz F, Nader F, Daemi M, Jang SL, Johnston N, Sharma S. Prevalence of known risk factors for type 2 diabetes mellitus in multiethnic urban youth in Edmonton: Findings from the WHY ACT NOW project. Can J Diabetes. 2019;43(3):207-14. [Link] [DOI:10.1016/j.jcjd.2018.10.002]
25. Lee JH, Kim YM, Kwak MJ, Kim SY, Kim HJ, Cheon CK, et al. Incidence trends and associated factors of diabetes mellitus in Korean children and adolescents: A retrospective cohort study in Busan and Gyeongnam. Ann Pediatr Endocrinol Metab. 2015;20(4):206-12. [Link] [DOI:10.6065/apem.2015.20.4.206]
26. Majeed AAS, Hassan K. Risk factors for type 1 diabetes mellitus among children and adolescents in Basrah. Oman Med J. 2011;26(3):189-95. [Link] [DOI:10.5001/omj.2011.46]
27. de Vasconcelos HC, de Araújo MF, Damasceno MM, de Almeida PC, de Freitas RW. Risk factors for type 2 diabetes mellitus among adolescents. Revista da Escola de Enfermagem da USP. 2010;44(4):881-7. [Portuguese] [Link] [DOI:10.1590/S0080-62342010000400004]
28. Young TK, Martens PJ, Taback SP, Sellers EAC, Dean HJ, Cheang M, et al. Type 2 diabetes mellitus in children: Prenatal and early infancy risk factors among native Canadians. Arch Pediatr Adolesc Med. 2002;156(7):651-5. [Link] [DOI:10.1001/archpedi.156.7.651]
29. Zamani M, Rahmanian V, Rezaei S, Khubfekr H, Namdar A. Factors associated with type-1 diabetes mellitus in children aged 2-15 years: A case-control study from southern Iran. Res Sq. 2021. [Link] [DOI:10.21203/rs.3.rs-250793/v1]
30. Barakat C, Yousufzai SJ, Booth A, Benova L. Prevalence of and risk factors for diabetes mellitus in the school-attending adolescent population of the United Arab Emirates: A large cross-sectional study. BMJ Open. 2021;11(9):e046956. [Link] [DOI:10.1136/bmjopen-2020-046956]
31. Bang KS, Jang SY, Choe JH. Factors affecting high-risk for diabetes among Korean adolescents: An analysis using the eighth Korea national health and nutrition examination survey (2020). Children. 2022;9(8):1249. [Link] [DOI:10.3390/children9081249]
32. Xie T, Hao L, Liu Y, Zhang D, Bi YW, Wang T, et al. Risk factor for diabetes mellitus in pediatric chronic pancreatitis patients. Medicine. 2019;98(48):e17984. [Link] [DOI:10.1097/MD.0000000000017984]
33. Ouyang A, Hu K, Chen L. Trends and risk factors of diabetes and prediabetes in US adolescents, 1999-2020. Diabetes Res Clin Pract. 2023;207:111022. [Link] [DOI:10.1016/j.diabres.2023.111022]
34. Lee PH. Association between adolescents' physical activity and sedentary behaviors with change in BMI and risk of type 2 diabetes. PLoS One. 2014;9(10):e110732. [Link] [DOI:10.1371/journal.pone.0110732]
35. Jaja T, Yarhere IE. Risk factors for type 2 diabetes mellitus in adolescents secondary school students in Port Harcourt, Nigeria. Niger J Paediatr. 2015;42(2):137-41. [Link] [DOI:10.4314/njp.v42i2.13]
36. Mahumud RA, Sahle BW, Owusu-Addo E, Chen W, Morton RL, Renzaho AMN. Association of dietary intake, physical activity, and sedentary behaviours with overweight and obesity among 282,213 adolescents in 89 low and middle income to high-income countries. Int J Obes. 2021;45(11):2404-18. [Link] [DOI:10.1038/s41366-021-00908-0]
37. Hilliard ME, Powell PW, Anderson BJ. Evidence-based behavioral interventions to promote diabetes management in children, adolescents, and families. Am Psychol. 2016;71(7):590-601. [Link] [DOI:10.1037/a0040359]
38. Standl E, Khunti K, Hansen TB, Schnell O. The global epidemics of diabetes in the 21st century: Current situation and perspectives. Eur J Prev Cardiol. 2019;26(Suppl 2):7-14. [Link] [DOI:10.1177/2047487319881021]
39. Besseling J, Kastelein JJP, Defesche JC, Hutten BA, Hovingh GK. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA. 2015;313(10):1029-36. [Link] [DOI:10.1001/jama.2015.1206]
40. Holtz CA, Fox RA, Meurer JR. Incidence of behavior problems in toddlers and preschool children from families living in poverty. J Psychol. 2015;149(1-2):161-74. [Link] [DOI:10.1080/00223980.2013.853020]
41. Anjana RM, Lakshminarayanan S, Deepa M, Farooq S, Pradeepa R, Mohan V. Parental history of type 2 diabetes mellitus, metabolic syndrome, and cardiometabolic risk factors in Asian Indian adolescents. Metabolism. 2009;58(3):344-50. [Link] [DOI:10.1016/j.metabol.2008.10.006]
42. Rodriguez-Moran M, Guerrero-Romero F. Hyperinsulinemia in healthy children and adolescents with a positive family history for type 2 diabetes. Pediatrics. 2006;118(5):e1516-22. [Link] [DOI:10.1542/peds.2006-0845]
43. Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: Epidemiology and treatment. Curr Diabetes Rep. 2014;14(8):508. [Link] [DOI:10.1007/s11892-014-0508-y]
44. Suglia SF, Demmer RT, Wahi R, Keyes KM, Koenen KC. Depressive symptoms during adolescence and young adulthood and the development of type 2 diabetes mellitus. Am J Epidemiol. 2016;183(4):269-76. [Link] [DOI:10.1093/aje/kwv149]
45. Valletta JJ, Chipperfield AJ, Clough GF, Byrne CD. Daily energy expenditure, cardiorespiratory fitness and glycaemic control in people with type 1 diabetes. PloS One. 2014;9(5):e97534. [Link] [DOI:10.1371/journal.pone.0097534]
46. Simon SL, Higgins J, Melanson E, Wright KP, Nadeau KJ. A model of adolescent sleep health and risk for type 2 diabetes. Curr Diabetes Rep. 2021;21(2):4. [Link] [DOI:10.1007/s11892-020-01373-1]
47. Eckert K. Impact of physical activity and bodyweight on health-related quality of life in people with type 2 diabetes. Diabetes Metab Syndr Obes. 2012;5:303-11. [Link] [DOI:10.2147/DMSO.S34835]
48. Fletcher EA, McNaughton SA, Crawford D, Cleland V, Della Gatta J, Hatt J, et al. Associations between sedentary behaviours and dietary intakes among adolescents. Public Health Nutr. 2018;21(6):1115-22. [Link] [DOI:10.1017/S136898001700372X]
49. Tremblay J, Hamet P. Environmental and genetic contributions to diabetes. Metabolism. 2019;100:153952. [Link] [DOI:10.1016/j.metabol.2019.153952]
50. Garber AJ. Obesity and type 2 diabetes: Which patients are at risk?. Diabetes Obes Metab. 2012;14(5):399- 408. [Link] [DOI:10.1111/j.1463-1326.2011.01536.x]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







