Volume 10, Issue 2 (2022)
Health Educ Health Promot 2022, 10(2): 411-421 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jasim O, Mahmood M, Ad'hiah A. Prevalence and Prediction of Prediabetes among Apparently Healthy Iraqis from Baghdad. Health Educ Health Promot 2022; 10 (2) :411-421
URL: http://hehp.modares.ac.ir/article-5-53715-en.html
URL: http://hehp.modares.ac.ir/article-5-53715-en.html
1- Department of Biology, College of Science, Mustansiriyah University, Baghdad, Iraq
2- Tropical-Biological Research Unit, College of Science, University of Baghdad, Baghdad, Iraq
2- Tropical-Biological Research Unit, College of Science, University of Baghdad, Baghdad, Iraq
Keywords: Prediabetic State [MeSH], Diabetes Mellitus [MeSH], C-Reactive Protein [MeSH], Interleukins [MeSH]
Full-Text [PDF 648 kb]
(3768 Downloads)
| Abstract (HTML) (1562 Views)
Table 1) Results of characteristics of normoglycemia, prediabetes, and diabetes mellitus participants

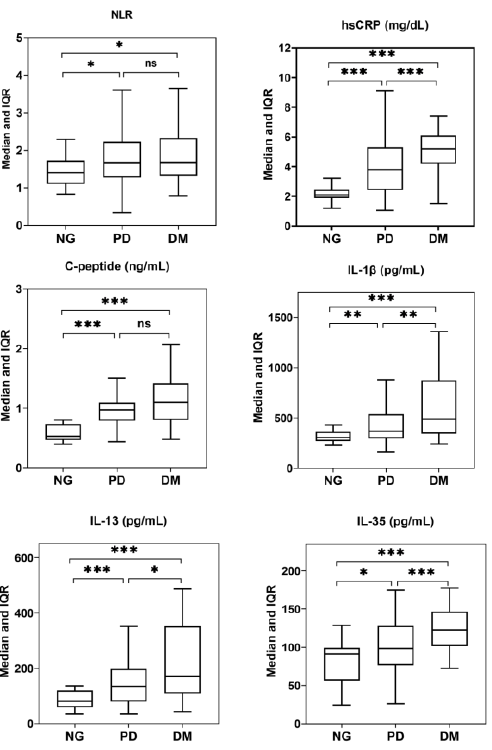
Figure 1) Box plot presentation of neutrophil-to-lymphocyte ratio (NLR), High-sensitivity C-reactive protein (hsCRP), C-peptide, IL-1β, IL-13, and IL-35 in normoglycemia (NG), prediabetes (PD) and diabetes mellitus (DM). *, ** and ***: Significant difference at the levels 0.05, 0.01 and 0.001, respectively. ns: Non-significant difference.
Table 2) ROC curve analysis of NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35 in prediabetes and diabetes mellitus

Table 3) Logistic regression analysis of NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35 in prediabetes and diabetes mellitus patients divided into three groups based on levels of each variable
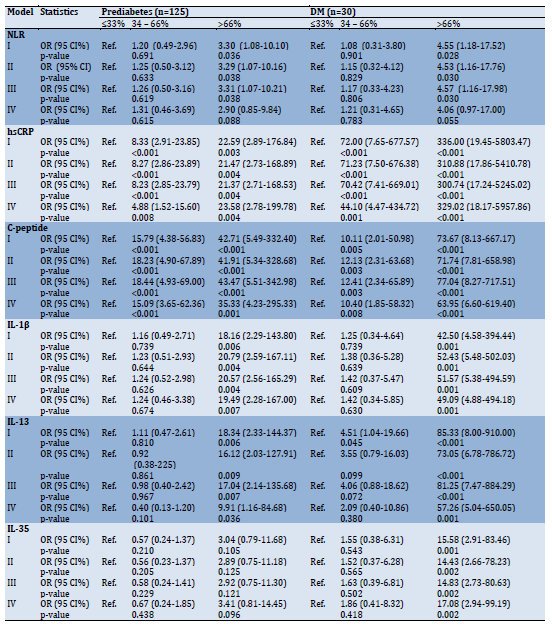
Table 4) Spearman's rank order correlation coefficients among study variables.

Discussion
It is universally recognized that prediabetes is an important metabolic condition, and besides putting individuals at risk of developing DM, individuals with prediabetes are at greater risk of developing many of the comorbidities typically associated with DM; for instance, neuropathy, retinopathy, nephropathy, and CVD [3]. Therefore, identification of prediabetes may aid in planning DM control programs and can contribute to a good quality of life and significantly reduce healthcare expenditures [25]. In the current study, 17.0% of 735 healthy individuals were identified as having prediabetes (about one in six adults), most of whom were older than 40 years and were overweight/obese. The prevalence of prediabetes varied in regional and Arab countries, and a recent review article described a relatively high prevalence in Iraq, Saudi Arabia, the United Arab Emirates and Kuwait (range: 19.3–28.6%), an intermediate prevalence in Iran and Qatar (range: 13.8–14.6%) and a low prevalence in Yemen and Syria Oman and Tunisia (range: 4.6-9.0%) [26]. In general, estimates of the prevalence of prediabetes vary worldwide and this is mainly related to the diagnostic criteria used (ADA or WHO guidelines), test selection (FPG, Hb1Ac, 2hPPG or all) and the ethnicity of the population studied [3]. For instance, A meta-analysis of 24 studies in Caucasian and Asian populations estimated the prevalence of impaired fasting glucose (IFG) to be 36.0 and 53.1% using WHO and ADA guidelines, respectively, while the use of two diagnostic tests (IFG and IGT) revealed prevalence rates of 15.8 and 20.2% for WHO and ADA guidelines, respectively [27].
Regardless of these differences in the prevalence of prediabetes, it is important to screen individuals at increased risk of prediabetes at an early stage to prepare for DM intervention and control measures. Among the risk factors identified in the current group of prediabetes are age and BMI. About 70.0% of patients were classified under the age range 40-74 years and more than 95% were either overweight or obese. Besides, triglyceride and VLDL levels were within the upper limit of the reference range. It has been found that prediabetes is more common in the elderly than in young adults, and in 2010, about 48% of American adults aged 65 and over had the condition. Furthermore, prediabetes is associated with higher mortality in the elderly [28]. These results can be expected as it has been reported that 92% of the elderly have at least one chronic disease and 77% have at least two chronic diseases such as CVD and DM [29]. In addition, the prevalence of overweight/obesity in the elderly continues to rise over time, and some data have estimated obesity rates to exceed 37.5% among males and 39.4% among females over the age of 60 [30]. Obesity is a global health epidemic that predisposes to DM and CVD, and studies have shown that obesity has a causal effect on DM. Besides, a significant causal effect of obesity on prediabetes and insulin resistance has also been identified among non-diabetic individuals [31]. It has also been indicated that older ages, obesity, and dysregulated levels of lipid-related parameters are interrelated. In a Chinese study, 6722 elderly subjects were included to assess the predictive ability of some risk factors in determining metabolic syndrome, including BMI, waist-to-height ratio, triglyceride-to-high-density-lipoprotein-cholesterol, lipid accumulation product, and visceral adiposity index. The results showed that these factors performed high predictive value in determining the metabolic syndrome [32]. A study of similar and other risk factors was performed in a cohort of 3,307 elderly Colombian individuals with prediabetes and found that the triglyceride-to-glucose fasting index had the best discriminatory power for predicting prediabetes in the entire population. Thus, the authors suggested that this indicator could be used as a complementary marker to assess prediabetes in the elderly [33].
The current study also targeted other factors that are proposed to have a role in predicting prediabetes. These were NLR, hsCRP, C-peptide, IL-1β, IL-13 and IL-35. The NLR, a newly identified inflammatory biomarker for DM, showed a similar significant elevation in both the prediabetes and DM groups compared to individuals with normoglycemia. These results are consistent with other reports that have shown higher NLR values in patients with prediabetes or DM and suggested that the NLR may reflect a systemic inflammatory response and could be considered a predictive and prognostic factor for diabetes and related complications [7, 8]. Regarding the predictive significance of NLR, ROC analysis of the present data revealed that NLR was a poor predictor of prediabetes or DM because the estimated AUC was in the range of 0.641–0.685. Besides, logistic regression analysis showed no significante difference of the NLR in the risk of developing prediabetes or diabetes. Therefore the increase in NLR may be related to other factors. Since neutrophils are the predominant leukocytes in peripheral blood that respond rapidly to inflammatory stimuli, their number would be expected to increase upon inflammatory responses. In addition, elevated levels of some pro-inflammatory cytokines may cause neutrophilia and lymphopenia and consequently an elevated NLR [7].
The study also showed that hsCRP and C-peptide levels were significantly elevated in the serum of prediabetes and DM patients. ROC curve analysis showed that both biomarkers were significant predictors of both conditions with AUCs of 0.829 and 0.961 for hsCRP and 0.913 and 0.892 for C-peptide in prediabetes and diabetes, respectively. Logistic regression analysis revealed that individuals in the third tertile of hsCRP and C-peptide levels (>66%) had a 23.58- and 35.33-fold increased risk of developing prediabetes, respectively, and this risk was higher in DM. Most of the published studies support these findings and confirm that hsCRP and C-peptide show up-regulated levels in patients with prediabetes or diabetes [10–13]. hsCRP is synthesized by hepatocytes in response to inflammatory stimuli and is thus considered a sensitive and systemic biomarker of inflammation, especially in diseases with low-grade chronic inflammation such as DM [9, 10]. hsCRP has also been linked to the risk of prediabetes, and patients with this condition showed significantly elevated levels of hsCRP in their serum [11]. It is interesting to note that a previous study showed that elevated levels of hsCRP were also associated with a higher risk of type 2 DM, but when categorized according to HbA1c levels, hsCRP was only positively associated with type 2 DM among those who had high levels of HbA1c (undiagnosed DM), but not low levels of HbA1c (incident DM). Therefore, elevated hsCRP levels have been suggested as byproducts of hyperglycemia, rather than directly contributing to the development of incident DM [34]. The association between hsCRP and cardiovascular events and all-cause mortality in patients with high-risk type 2 DM was also evaluated, and hsCRP was proposed as an independent risk factor for this cardiovascular complication in patients [35].
The current study revealed that C- peptide can be considered an important and relevant predictor of prediabetes and DM. C-peptide is a part of pro-insulin that is cleaved before co-secreting with insulin from pancreatic beta-cells. It is considered a useful biomarker and is superior to insulin in assessing the function of these cells because the C-peptide half-life is longer than that of insulin (20-30 vs. 3-5 minutes) and hepatic clearance is minimal compared to insulin [12]. Therefore, studies have suggested that C-peptide would be more effective in the prediction of prediabetes and DM compared to insulin [13, 36, 37]. There is also evidence to suggest that the C-peptidogenic index (C-peptide increase by glucose loading) is the most independent predictor of future DM. Further, the index was more correlated with beta-cell mass and function than the insulin-based index [38]. In addition, A positive association was found between C-peptide and the risk of developing prediabetes and DM among Chinese women with a history of gestational diabetes [13].
The final part of this study addressed the role of three cytokines in predicting prediabetes and DM. One of them is a pro-inflammatory cytokine (IL-1β), while the other two are anti-inflammatory cytokines (IL-13 and IL-35). These cytokines showed up-regulated levels in serum of prediabetes and DM patients, but the ROC curve analysis revealed that they were nearly poor predictors of prediabetes (AUC=0.672, 0.728, and 0.631, respectively), while their prognostic significance was indicated in DM (AUC=0.804, 0.868 and 0.833, respectively). Elevated levels of IL-1β were associated with an increased risk of prediabetes and DM (OR=19.49 and 49.09, respectively). Consistent with these findings, animal model studies and clinical trials have provided evidence supporting the causative role of IL-1β as an essential factor in the loss of beta-cell mass in type 2 DM. In addition, an IL-1β-mediated autoinflammatory process that leads to beta-cell death has been demonstrated [39]. It has also been found that beta-cells can produce IL-1β when stimulated with glucose and this leads to the attraction of macrophages that can contribute to increased production of IL-1β [40]. IL-1β has also been hypothesized to be a potential candidate for promoting progression from prediabetes to overt DM [16]. The present data are in good agreement with this hypothesis as IL-1β levels showed a significant increase in prediabetes compared to normoglycemia but the increase was higher in DM and the difference was significant compared to prediabetes. More supporting evidence has been recently addressed and IL-1β was considered an important predictor of new prediabetes in patients with acute pancreatitis [17]. Because of these effects of IL-1β, this cytokine has been a therapeutic target in DM and it has been indicated that anti-diabetic therapies should not only correct hyperglycemia but reduce inflammation. One advanced treatment that counteracts the pathophysiological effects of IL-1β is anakinra, and some promising results have been shown [41]. In a mouse model of gestational diabetes, increased expression of IL-1β was found in the uterus and the placenta along with elevated IL-1β serum levels compared to pregnant mice with normoglycemia. Treatment with anti-IL-1β antibodies improved glucose tolerance in mice with gestational diabetes without apparent adverse effects on the fetus [42].
As in IL-1β, the anti-inflammatory cytokine IL-13 is suggested to increase the risk of prediabetes and DM (OR=9.91 and 57.26, respectively). The results of previous studies have been conflicting regarding this issue. Serum IL-13 levels were significantly elevated in insulin-resistant patients compared to non-insulin-resistant controls [19] and in type 2 DM patients relative to controls [43]. IL-13 levels were also elevated in newly diagnosed tuberculosis patients with prediabetes [44]. In contrast, elevated levels of IL-13 have been associated with a lower risk of prediabetes, incident type 2 DM, and need for insulin therapy [20], whereas in patients with schizophrenia, serum IL-13 levels did not show significant differences between the prediabetes group and normal glucose tolerant group [45]. In patients with type 1 DM, IL-13 production showed significantly lower levels even in those with a genetic risk for disease progression, compared to healthy control individuals. In this context, IL-13 has been suggested to be an inhibitor of beta-cell destruction and is considered a key regulator of glucose metabolism [22]. Apart from these conflicting results, IL-13 appears to be an important target of research aimed at understanding the pathogenesis of DM. The increased serum level of IL-13 in current prediabetes and DM patients may be indicative of the highly complex interplay between this cytokine and its pro-inflammatory counterparts such as IL-1β. Elevated levels of IL-13 may indicate its role as a compensatory and anti-inflammatory regulatory molecule that may limit the damage caused by increased levels of pro-inflammatory cytokines [43].
The second anti-inflammatory cytokine is IL-35 and the present results indicate up-regulated levels of this cytokine in prediabetes and DM patients. ROC curve analysis revealed that IL-35 is more predictive of DM than prediabetes (AUC=0.833 and 0.631, respectively). Logistic regression analysis also confirmed that patients in the third tertile of IL-35 level (>66%) were 17.08-fold more likely to develop DM, while this increased risk was not observed in patients with prediabetes (odds ratio=3.41; p=0.096). IL-35 has not been well examined in DM and there was probably only one study that evaluated serum levels in type 2 DM patients with and without chronic periodontitis and found that both pathological conditions did not affect IL-35 levels [24]. In a rat model of DNP, intrathecal injection of IL-35 had anti-inflammatory and anti-apoptotic effects and was associated with a lower accumulation of pro-inflammatory cytokines in the spinal cord [23]. Further, low levels of IL-35 have been indicated to play a pivotal role in the development of type 1 DM and the authors recommended that the therapeutic potential of IL-35 be studied in type 1 DM [22]. Other inflammatory and autoimmune diseases are generally associated with low levels of IL-35; for instance, rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), but observations have been inconsistent [46]. In a study, the IL-35 was up-regulated at sites of inflammation in patients with RA and proposed its pro-inflammatory potential [47]. High levels of plasma IL-35 were also found in patients with active SLE who had low expression of the IL-35 receptor (gp130) on CD4+ Th cells [48]. It has been hypothesized that increased expression of IL-35 may indicate an immune-regulating association between CD4+ effector T cells and Treg cells in response to an intense inflammatory environment. Indeed, studies showed that expression of Ebi3 and p35 (IL-35 subunits) was significantly elevated in Treg cells when co-cultured with CD4+ effector T cells. This may indicate that presence of the latter cells is responsible for the conduction of IL-35 synthesis signals [46]. A recent study found that the percentage of effector memory T cells was significantly increased in type 2 DM especially in those with CVD [49]. The involvement of IL-35 in the pathogenesis of DM appears to be complex and more simultaneous evaluations, such as effector T cells, Treg cells and the IL-35 receptor, are needed.
Spearman's rank order correlation analysis between the study variables showed some significant positive correlations. Interestingly, FPG was correlated with NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35, and similar correlations were shown with Hb1Ac except for the NLR. These results suggest a causal relationship between these variables, and hyperglycemia, as measured by FPG and Hb1Ac, may affect immune homeostasis and thus may alter the pro-inflammatory and anti-inflammatory environment in the human body. In this context, pro-inflammatory and anti-inflammatory cytokines have been proposed to be involved in DM progression, capable of interfering with insulin-responsive glucose uptake and inducing insulin resistance [50].
Regardless of the significance of these findings, some limitations should be addressed. First, the number of participants was relatively small compared to other published studies. In addition, the results of this study cannot be generalized to the rest of the Iraqi governorates because the participants were from Baghdad only. Second, only two tests (FPG and Hb1Ac) were used to define prediabetes, and these tests should be accompanied by the 2hPPG test. Third, WHO guidelines were followed to identify overweight/obese individuals, and these guidelines may have a different profile in Iraqis.
Conclusion
The prevalence of prediabetes among apparently healthy Iraqi individuals was 17%. Prediabetes was more prevalent in individuals over 40 years of age, especially those who were overweight/obese. Pro-inflammatory and anti-inflammatory cytokines were up-regulated in serum of prediabetes patients.
Acknowledgments: The authors appreciate the cooperation of the medical staff at the Primary Health Care Center in Baghdad.
Ethical Permissions: The Ethics Committee of the Iraqi Ministry of Health and Environment approved the study protocol.
Conflicts of Interests: None declared.
Authors’ Contributions: Jasim OH (First Author), Introduction Writer/Main Researcher/Statistical Analyst/
Discussion Writer (34%); Mahmood MM (Second Author), Assistant Researcher (33%); Ad'hiah AH (Third Author), Methodologist (33%)
Funding/Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Full-Text: (978 Views)
Introduction
Diabetes mellitus (DM) is a disorder of glucose metabolism and based on data published between 1990 and 2018, the prevalence of DM was estimated at 9.3% and is expected to rise to 10.2% by 2030 and 10.9% by 2045 [1]. These data suggest that DM represents a health problem of global public concern and has a significant impact on the well-being of affected individuals as well as their families and communities [2]. Besides, DM patients are more likely to develop cardiovascular disease (CVD) and microvascular complications, which may already be present before diagnosis. Therefore, it is very important to detect people at risk of developing DM at an early stage (i.e. identify individuals with prediabetes).
Prediabetes is a chronic metabolic condition characterized by intermediate hyperglycemia in which blood glucose levels are above normal and below the diagnostic threshold for DM [3]. Data on the prevalence of prediabetes reported in the literature vary widely and lack comprehensive global prevalence estimates [4]. In 2019, based on impaired glucose tolerance (IGT), the prevalence of prediabetes was estimated at 7.5% and is expected to reach 8.0% by 2030 and 8.6% by 2045 [1]. Various guidelines have been developed to identify patients with prediabetes, but in general, three laboratory evaluations are performed; fasting plasma glucose (FPG), glycosylated hemoglobin (HbA1c), and two-hour post-load plasma glucose (2hPPG) or all [5].
The progression from prediabetes to DM is a complex and multifactorial process with many operational indicators described as playing a role such as age, gender, body mass index (BMI), waist circumference, blood pressure, ethnicity, and family history of DM [6]. Other risk factors, particularly those related to inflammation, have also been proposed to predict the progression of prediabetes to DM; for instance neutrophil-to-lymphocyte ratio (NLR), high-sensitivity C-reactive protein (hsCRP), C-peptide and pro-inflammatory and anti-inflammatory cytokines, but the evidence has not been conclusive. NLR is an inflammatory marker obtained simply by dividing the absolute count of neutrophils by the absolute count of lymphocytes. This marker has been linked to a risk of certain inflammatory and autoimmune diseases, including DM. Besides, an elevated NLR in healthy subjects is suggested to be an indicator of impaired glucose metabolism [7, 8]. CRP, another inflammatory marker, is an acute phase protein that is elevated in the serum of patients with infectious and inflammatory conditions. hsCRP is the highly sensitive form of CRP and can predict low-grade inflammation in the absence of obvious inflammation [9]. It has been indicated that hsCRP can be considered a reliable indicator of prediabetes, as well as obesity and abnormal lipid metabolism in young adults [10]. In the adult population, elevated levels of hsCRP have also been associated with the risk of prediabetes, in addition to being correlated with overweight/obesity and higher levels of lipid profile parameters [11]. C-peptide is an important marker widely used to assess pancreatic beta-cell function, and there is increasing evidence to suggest that this marker is a useful predictor of glycemic control and response to hypoglycemic treatments, as well as the risk of future complications in patients with DM [12]. Further, a positive association between serum C-peptide levels and risk of DM and prediabetes was indicated among women with a history of gestational diabetes [13].
About cytokines, there is clear evidence that pro-inflammatory and anti-inflammatory cytokines significantly contribute to the development of abnormal glucose and lipid metabolism, suggesting a role in the risk of prediabetes and DM [14]. Among these cytokines are interleukin (IL)-1β, IL-13 and IL-35. IL-1β, a member of the IL-1 cytokine family, is a pro-inflammatory cytokine with diverse physiological and pathological functions and plays an important role in mediating inflammatory responses in a wide range of diseases [15]. In DM, and since the disease is characterized by a chronic, low-grade inflammatory state, it has been proposed that IL-1β can enhance inflammatory signaling and thus contribute to the development of DM and its associated complications [16]. Importantly, it has recently been shown that elevated levels of IL-1β significantly predicted the onset of new prediabetes after acute pancreatitis [17]. IL-13 is a distinct cytokine of T helper (h) 2 cells with anti-inflammatory effects, and in recent years, its role in the pathogenesis of many inflammatory and autoimmune diseases, including type 1 DM, has been increasingly recognized [18]. In the case of prediabetes and type 2 DM, the role of IL-13 needs to be clarified as inconsistent observations have been reported [19, 20]. IL-35 is the newest member of the IL-12 family of cytokines primarily expressed by regulatory T cells (Tregs) and known to display immunosuppressive activities [21]. The importance of this cytokine in DM has not been well demonstrated, but its role in the risk of type 1 DM has been suggested and may be considered a therapeutic strategy [22]. In a rat model of diabetic neuropathic pain (DNP), IL-35 was associated with reduced progression of inflammation [23]. However, serum levels of IL-35 were not affected in patients with type 2 DM [24].
The current study sought to determine the prevalence of prediabetes in a cohort of apparently healthy Iraqi individuals using the American Diabetes Association (ADA) criteria, which are FPG and HbA1c. Besides, the study aimed to evaluate the significance of NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35 in predicting prediabetes.
Materials and Methods
A cross-sectional, case-control study was conducted from January to July 2021 to determine the prevalence of prediabetes and to assess the significance of NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35 as predictors of prediabetes. The study included 735 apparently healthy subjects who voluntarily visited a Primary Health Care Center in Baghdad for basic laboratory testing (59.5% males; age range: 18-76 years). Of the 735 apparently healthy subjects, 30 were randomly selected and considered a control group or a normoglycemic group according to the ADA criteria (FPG: <100 mg/dL; HbA1c: <5.7%). Both groups (DM and normoglycemia) were also evaluated for the parameters of prediabetes. According to the ADA criteria, prediabetes was defined as FPG of 100-125mg/dL and HbA1c of 5.7-6.4%. Participants who followed these criteria were considered prediabetes patients and their age, gender body mass index (BMI), and waist circumference were recorded. Besides, their blood was tested for complete blood count (CBC), total cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP). Blood samples were collected after fasting for 12-14 hours. Thirty newly diagnosed cases with DM were also included. They followed the ADA criteria (FPG: 126 mg/dL or higher; HbA1c: 6.5% or higher) and were not on any anti-diabetic medications. They were referred to the Primary Health Care Center on suspicion of having diabetes due to signs and symptoms of diabetes; for instance, abnormal thirst, frequent urination, and weight loss.
The Iraqi Ministry of Health and Environment Ethics Committee approved the study protocol. All participants were informed of the nature of the study and provided written consent. CBC was determined using the CELL-DYN Emerald Hematology Analyzer (Abbott, U.S.A). NLR was determined by dividing the absolute count of neutrophils by the absolute count of lymphocytes. Cobas c 311analyzer (Cobas-Roche, Germany) preloaded with the required reagents was used to assess FPG, HbA1c, total cholesterol, triglycerides, HDL, LDL, VLDL, ALT, AST, and ALP. Serum levels of hsCRP, C-peptide, IL-1β, IL-13, and IL-35 were determined using enzyme-linked immunosorbent assay (ELISA) kits, and instructions of manufacture were followed (Cusabio Technology LLC, USA).
Categorical variables were given as numbers and percentages, and the Pearson Chi-square test was used to assess significant differences. Two tests of normality were applied to continuous variables; Kolmogorov-Smirnov and Shapiro-Wilk tests. Normally distributed variables (parametric variables) were expressed as mean and standard deviation (SD), and significant differences were assessed using one-way analysis of variance (ANOVA) post hoc Duncan's multiple range test. Skewed variables (non-parametric variables) were identified by the median and interquartile range (IQR: 25-75%), and significant differences were assessed using the Mann-Whitney U test (to compare two variables) or Kruskal-Wallis test (to compare more than two variables). Receiver operating characteristic (ROC) curve analysis was performed to determine the area under the curve (AUC), 95% confidence interval (CI), cut-off value (optimized with Youden index), sensitivity, and specificity. Logistic regression analysis was applied to determine the odds ratio (OR) and 95% CI. In this analysis, participants were divided into three groups based on the levels of each variable (tertiles: ≤33%, 34–66%, >66%). Besides, four models were adopted to perform the analysis; I (unadjusted), II (age-adjusted), III (age- and gender-adjusted), and IV (age-, gender- and BMI-adjusted). The relationship between variables was assessed using Spearman's rank-order correlation analysis. A probability (p) value ≤0.05 was taken as statistically significant. IBM SPSS Statistics 25.0 (Armonk, NY: IBM Corp.) and GraphPad Prism version 8.0.0 (San Diego, California USA) were used to perform statistical analysis.
Findings
Of the 735 participants, 125 (17.0%) were considered prediabetes after applying the ADA criteria; FPG was 108.4±6.0 mg/dL and Hb1Ac was 6.0±0.4%. Both variables were significantly higher than in normoglycemic individuals and significantly lower than in DM patients. A roughly similar observation was made when mean age was examined in prediabetes, DM, and normoglycemia. In terms of age groups, although there were no significant differences between normoglycemia, prediabetes, and DM, most prediabetes and DM patients were older than 40 years (about 70.0%). However, there were no gender-related differences between the three groups. In the case of BMI, most prediabetes and DM patients were either overweight or obese and compared to normoglycemic individuals the difference was significant. Besides, waist circumference was significantly higher in prediabetes and DM than in normoglycemia. Hemoglobin and platelet count did not show any significant differences between the three groups, but white blood cell (WBC) count was significantly higher in prediabetes and DM groups than in the normoglycemia group, and this rise was more pronounced in neutrophil count. Serum levels of triglycerides, VLDL, and ALP were significantly higher in prediabetes and DM groups than in the normoglycemia group. ALT levels were significantly elevated in only prediabetes compared to normoglycemia and DM. The remaining variables (total cholesterol, HDL, LDL, and AST) did not show any significant differences between the three groups (Table 1).
As shown in Figure 1, medians of NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35 were significantly elevated in prediabetes and DM groups compared to the normoglycemia group, respectively. The elevation of these medians was greater in the DM group than in the prediabetes group except for NLR, where the difference was not significant.
ROC curve analysis revealed that the six variables examined (NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35) could be considered important biomarkers in distinguishing between prediabetes or DM and normoglycemia, but the AUC value was different and this was dependent on disease status (prediabetes or DM) and the variable under investigation. In prediabetes, C-peptide recorded the highest AUC value followed by hsCRP, IL-13, IL-1β, NLR, and IL-35. In DM, the pattern was different and hsCRP recorded the highest AUC followed by C-peptide, IL-13, IL-35, IL-1β, and NL (Table 2).
Multinomial regression analysis was conducted after participants were divided into three groups based on levels of NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35 (tertiles: ≤33%, 34–66%, >66%); group ≤33% was the reference category. The analysis was performed under four models; I (unadjusted), II (age-adjusted), III (age- and gender-adjusted), and IV (age-, gender- and BMI-adjusted) and since there were no obvious differences between the analyzes in these models, only the results of model IV will be illustrated. NLR was not associated with the risk of prediabetes or DM. hsCRP was associated with an increased risk of prediabetes or DM, especially in the >66% group. A similar trend was found in C-peptide. For IL-1β and IL-13, only individuals in the >66% group were most likely to have prediabetes or DM. In the case of IL-35, it was not associated with the risk of prediabetes, but in DM, individuals in the >66% group were at significantly increased risk (Table 3).
Significant positive correlations included age with hsCRP and IL-13, BMI with NLR, C-peptide and IL-13, FPG with NLR, hsCRP, C-peptide, IL-1β, IL-13 and IL-35, Hb1Ac with hsCRP, C-peptide, IL-1β, IL-13 and IL-35, triglycerides and VLDL with hsCRP, C-peptide and IL-13, and AST and ALP with hsCRP and C-peptide (Table 4).
Diabetes mellitus (DM) is a disorder of glucose metabolism and based on data published between 1990 and 2018, the prevalence of DM was estimated at 9.3% and is expected to rise to 10.2% by 2030 and 10.9% by 2045 [1]. These data suggest that DM represents a health problem of global public concern and has a significant impact on the well-being of affected individuals as well as their families and communities [2]. Besides, DM patients are more likely to develop cardiovascular disease (CVD) and microvascular complications, which may already be present before diagnosis. Therefore, it is very important to detect people at risk of developing DM at an early stage (i.e. identify individuals with prediabetes).
Prediabetes is a chronic metabolic condition characterized by intermediate hyperglycemia in which blood glucose levels are above normal and below the diagnostic threshold for DM [3]. Data on the prevalence of prediabetes reported in the literature vary widely and lack comprehensive global prevalence estimates [4]. In 2019, based on impaired glucose tolerance (IGT), the prevalence of prediabetes was estimated at 7.5% and is expected to reach 8.0% by 2030 and 8.6% by 2045 [1]. Various guidelines have been developed to identify patients with prediabetes, but in general, three laboratory evaluations are performed; fasting plasma glucose (FPG), glycosylated hemoglobin (HbA1c), and two-hour post-load plasma glucose (2hPPG) or all [5].
The progression from prediabetes to DM is a complex and multifactorial process with many operational indicators described as playing a role such as age, gender, body mass index (BMI), waist circumference, blood pressure, ethnicity, and family history of DM [6]. Other risk factors, particularly those related to inflammation, have also been proposed to predict the progression of prediabetes to DM; for instance neutrophil-to-lymphocyte ratio (NLR), high-sensitivity C-reactive protein (hsCRP), C-peptide and pro-inflammatory and anti-inflammatory cytokines, but the evidence has not been conclusive. NLR is an inflammatory marker obtained simply by dividing the absolute count of neutrophils by the absolute count of lymphocytes. This marker has been linked to a risk of certain inflammatory and autoimmune diseases, including DM. Besides, an elevated NLR in healthy subjects is suggested to be an indicator of impaired glucose metabolism [7, 8]. CRP, another inflammatory marker, is an acute phase protein that is elevated in the serum of patients with infectious and inflammatory conditions. hsCRP is the highly sensitive form of CRP and can predict low-grade inflammation in the absence of obvious inflammation [9]. It has been indicated that hsCRP can be considered a reliable indicator of prediabetes, as well as obesity and abnormal lipid metabolism in young adults [10]. In the adult population, elevated levels of hsCRP have also been associated with the risk of prediabetes, in addition to being correlated with overweight/obesity and higher levels of lipid profile parameters [11]. C-peptide is an important marker widely used to assess pancreatic beta-cell function, and there is increasing evidence to suggest that this marker is a useful predictor of glycemic control and response to hypoglycemic treatments, as well as the risk of future complications in patients with DM [12]. Further, a positive association between serum C-peptide levels and risk of DM and prediabetes was indicated among women with a history of gestational diabetes [13].
About cytokines, there is clear evidence that pro-inflammatory and anti-inflammatory cytokines significantly contribute to the development of abnormal glucose and lipid metabolism, suggesting a role in the risk of prediabetes and DM [14]. Among these cytokines are interleukin (IL)-1β, IL-13 and IL-35. IL-1β, a member of the IL-1 cytokine family, is a pro-inflammatory cytokine with diverse physiological and pathological functions and plays an important role in mediating inflammatory responses in a wide range of diseases [15]. In DM, and since the disease is characterized by a chronic, low-grade inflammatory state, it has been proposed that IL-1β can enhance inflammatory signaling and thus contribute to the development of DM and its associated complications [16]. Importantly, it has recently been shown that elevated levels of IL-1β significantly predicted the onset of new prediabetes after acute pancreatitis [17]. IL-13 is a distinct cytokine of T helper (h) 2 cells with anti-inflammatory effects, and in recent years, its role in the pathogenesis of many inflammatory and autoimmune diseases, including type 1 DM, has been increasingly recognized [18]. In the case of prediabetes and type 2 DM, the role of IL-13 needs to be clarified as inconsistent observations have been reported [19, 20]. IL-35 is the newest member of the IL-12 family of cytokines primarily expressed by regulatory T cells (Tregs) and known to display immunosuppressive activities [21]. The importance of this cytokine in DM has not been well demonstrated, but its role in the risk of type 1 DM has been suggested and may be considered a therapeutic strategy [22]. In a rat model of diabetic neuropathic pain (DNP), IL-35 was associated with reduced progression of inflammation [23]. However, serum levels of IL-35 were not affected in patients with type 2 DM [24].
The current study sought to determine the prevalence of prediabetes in a cohort of apparently healthy Iraqi individuals using the American Diabetes Association (ADA) criteria, which are FPG and HbA1c. Besides, the study aimed to evaluate the significance of NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35 in predicting prediabetes.
Materials and Methods
A cross-sectional, case-control study was conducted from January to July 2021 to determine the prevalence of prediabetes and to assess the significance of NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35 as predictors of prediabetes. The study included 735 apparently healthy subjects who voluntarily visited a Primary Health Care Center in Baghdad for basic laboratory testing (59.5% males; age range: 18-76 years). Of the 735 apparently healthy subjects, 30 were randomly selected and considered a control group or a normoglycemic group according to the ADA criteria (FPG: <100 mg/dL; HbA1c: <5.7%). Both groups (DM and normoglycemia) were also evaluated for the parameters of prediabetes. According to the ADA criteria, prediabetes was defined as FPG of 100-125mg/dL and HbA1c of 5.7-6.4%. Participants who followed these criteria were considered prediabetes patients and their age, gender body mass index (BMI), and waist circumference were recorded. Besides, their blood was tested for complete blood count (CBC), total cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP). Blood samples were collected after fasting for 12-14 hours. Thirty newly diagnosed cases with DM were also included. They followed the ADA criteria (FPG: 126 mg/dL or higher; HbA1c: 6.5% or higher) and were not on any anti-diabetic medications. They were referred to the Primary Health Care Center on suspicion of having diabetes due to signs and symptoms of diabetes; for instance, abnormal thirst, frequent urination, and weight loss.
The Iraqi Ministry of Health and Environment Ethics Committee approved the study protocol. All participants were informed of the nature of the study and provided written consent. CBC was determined using the CELL-DYN Emerald Hematology Analyzer (Abbott, U.S.A). NLR was determined by dividing the absolute count of neutrophils by the absolute count of lymphocytes. Cobas c 311analyzer (Cobas-Roche, Germany) preloaded with the required reagents was used to assess FPG, HbA1c, total cholesterol, triglycerides, HDL, LDL, VLDL, ALT, AST, and ALP. Serum levels of hsCRP, C-peptide, IL-1β, IL-13, and IL-35 were determined using enzyme-linked immunosorbent assay (ELISA) kits, and instructions of manufacture were followed (Cusabio Technology LLC, USA).
Categorical variables were given as numbers and percentages, and the Pearson Chi-square test was used to assess significant differences. Two tests of normality were applied to continuous variables; Kolmogorov-Smirnov and Shapiro-Wilk tests. Normally distributed variables (parametric variables) were expressed as mean and standard deviation (SD), and significant differences were assessed using one-way analysis of variance (ANOVA) post hoc Duncan's multiple range test. Skewed variables (non-parametric variables) were identified by the median and interquartile range (IQR: 25-75%), and significant differences were assessed using the Mann-Whitney U test (to compare two variables) or Kruskal-Wallis test (to compare more than two variables). Receiver operating characteristic (ROC) curve analysis was performed to determine the area under the curve (AUC), 95% confidence interval (CI), cut-off value (optimized with Youden index), sensitivity, and specificity. Logistic regression analysis was applied to determine the odds ratio (OR) and 95% CI. In this analysis, participants were divided into three groups based on the levels of each variable (tertiles: ≤33%, 34–66%, >66%). Besides, four models were adopted to perform the analysis; I (unadjusted), II (age-adjusted), III (age- and gender-adjusted), and IV (age-, gender- and BMI-adjusted). The relationship between variables was assessed using Spearman's rank-order correlation analysis. A probability (p) value ≤0.05 was taken as statistically significant. IBM SPSS Statistics 25.0 (Armonk, NY: IBM Corp.) and GraphPad Prism version 8.0.0 (San Diego, California USA) were used to perform statistical analysis.
Findings
Of the 735 participants, 125 (17.0%) were considered prediabetes after applying the ADA criteria; FPG was 108.4±6.0 mg/dL and Hb1Ac was 6.0±0.4%. Both variables were significantly higher than in normoglycemic individuals and significantly lower than in DM patients. A roughly similar observation was made when mean age was examined in prediabetes, DM, and normoglycemia. In terms of age groups, although there were no significant differences between normoglycemia, prediabetes, and DM, most prediabetes and DM patients were older than 40 years (about 70.0%). However, there were no gender-related differences between the three groups. In the case of BMI, most prediabetes and DM patients were either overweight or obese and compared to normoglycemic individuals the difference was significant. Besides, waist circumference was significantly higher in prediabetes and DM than in normoglycemia. Hemoglobin and platelet count did not show any significant differences between the three groups, but white blood cell (WBC) count was significantly higher in prediabetes and DM groups than in the normoglycemia group, and this rise was more pronounced in neutrophil count. Serum levels of triglycerides, VLDL, and ALP were significantly higher in prediabetes and DM groups than in the normoglycemia group. ALT levels were significantly elevated in only prediabetes compared to normoglycemia and DM. The remaining variables (total cholesterol, HDL, LDL, and AST) did not show any significant differences between the three groups (Table 1).
As shown in Figure 1, medians of NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35 were significantly elevated in prediabetes and DM groups compared to the normoglycemia group, respectively. The elevation of these medians was greater in the DM group than in the prediabetes group except for NLR, where the difference was not significant.
ROC curve analysis revealed that the six variables examined (NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35) could be considered important biomarkers in distinguishing between prediabetes or DM and normoglycemia, but the AUC value was different and this was dependent on disease status (prediabetes or DM) and the variable under investigation. In prediabetes, C-peptide recorded the highest AUC value followed by hsCRP, IL-13, IL-1β, NLR, and IL-35. In DM, the pattern was different and hsCRP recorded the highest AUC followed by C-peptide, IL-13, IL-35, IL-1β, and NL (Table 2).
Multinomial regression analysis was conducted after participants were divided into three groups based on levels of NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35 (tertiles: ≤33%, 34–66%, >66%); group ≤33% was the reference category. The analysis was performed under four models; I (unadjusted), II (age-adjusted), III (age- and gender-adjusted), and IV (age-, gender- and BMI-adjusted) and since there were no obvious differences between the analyzes in these models, only the results of model IV will be illustrated. NLR was not associated with the risk of prediabetes or DM. hsCRP was associated with an increased risk of prediabetes or DM, especially in the >66% group. A similar trend was found in C-peptide. For IL-1β and IL-13, only individuals in the >66% group were most likely to have prediabetes or DM. In the case of IL-35, it was not associated with the risk of prediabetes, but in DM, individuals in the >66% group were at significantly increased risk (Table 3).
Significant positive correlations included age with hsCRP and IL-13, BMI with NLR, C-peptide and IL-13, FPG with NLR, hsCRP, C-peptide, IL-1β, IL-13 and IL-35, Hb1Ac with hsCRP, C-peptide, IL-1β, IL-13 and IL-35, triglycerides and VLDL with hsCRP, C-peptide and IL-13, and AST and ALP with hsCRP and C-peptide (Table 4).
Table 1) Results of characteristics of normoglycemia, prediabetes, and diabetes mellitus participants


Figure 1) Box plot presentation of neutrophil-to-lymphocyte ratio (NLR), High-sensitivity C-reactive protein (hsCRP), C-peptide, IL-1β, IL-13, and IL-35 in normoglycemia (NG), prediabetes (PD) and diabetes mellitus (DM). *, ** and ***: Significant difference at the levels 0.05, 0.01 and 0.001, respectively. ns: Non-significant difference.
Table 2) ROC curve analysis of NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35 in prediabetes and diabetes mellitus

Table 3) Logistic regression analysis of NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35 in prediabetes and diabetes mellitus patients divided into three groups based on levels of each variable

Table 4) Spearman's rank order correlation coefficients among study variables.

Discussion
It is universally recognized that prediabetes is an important metabolic condition, and besides putting individuals at risk of developing DM, individuals with prediabetes are at greater risk of developing many of the comorbidities typically associated with DM; for instance, neuropathy, retinopathy, nephropathy, and CVD [3]. Therefore, identification of prediabetes may aid in planning DM control programs and can contribute to a good quality of life and significantly reduce healthcare expenditures [25]. In the current study, 17.0% of 735 healthy individuals were identified as having prediabetes (about one in six adults), most of whom were older than 40 years and were overweight/obese. The prevalence of prediabetes varied in regional and Arab countries, and a recent review article described a relatively high prevalence in Iraq, Saudi Arabia, the United Arab Emirates and Kuwait (range: 19.3–28.6%), an intermediate prevalence in Iran and Qatar (range: 13.8–14.6%) and a low prevalence in Yemen and Syria Oman and Tunisia (range: 4.6-9.0%) [26]. In general, estimates of the prevalence of prediabetes vary worldwide and this is mainly related to the diagnostic criteria used (ADA or WHO guidelines), test selection (FPG, Hb1Ac, 2hPPG or all) and the ethnicity of the population studied [3]. For instance, A meta-analysis of 24 studies in Caucasian and Asian populations estimated the prevalence of impaired fasting glucose (IFG) to be 36.0 and 53.1% using WHO and ADA guidelines, respectively, while the use of two diagnostic tests (IFG and IGT) revealed prevalence rates of 15.8 and 20.2% for WHO and ADA guidelines, respectively [27].
Regardless of these differences in the prevalence of prediabetes, it is important to screen individuals at increased risk of prediabetes at an early stage to prepare for DM intervention and control measures. Among the risk factors identified in the current group of prediabetes are age and BMI. About 70.0% of patients were classified under the age range 40-74 years and more than 95% were either overweight or obese. Besides, triglyceride and VLDL levels were within the upper limit of the reference range. It has been found that prediabetes is more common in the elderly than in young adults, and in 2010, about 48% of American adults aged 65 and over had the condition. Furthermore, prediabetes is associated with higher mortality in the elderly [28]. These results can be expected as it has been reported that 92% of the elderly have at least one chronic disease and 77% have at least two chronic diseases such as CVD and DM [29]. In addition, the prevalence of overweight/obesity in the elderly continues to rise over time, and some data have estimated obesity rates to exceed 37.5% among males and 39.4% among females over the age of 60 [30]. Obesity is a global health epidemic that predisposes to DM and CVD, and studies have shown that obesity has a causal effect on DM. Besides, a significant causal effect of obesity on prediabetes and insulin resistance has also been identified among non-diabetic individuals [31]. It has also been indicated that older ages, obesity, and dysregulated levels of lipid-related parameters are interrelated. In a Chinese study, 6722 elderly subjects were included to assess the predictive ability of some risk factors in determining metabolic syndrome, including BMI, waist-to-height ratio, triglyceride-to-high-density-lipoprotein-cholesterol, lipid accumulation product, and visceral adiposity index. The results showed that these factors performed high predictive value in determining the metabolic syndrome [32]. A study of similar and other risk factors was performed in a cohort of 3,307 elderly Colombian individuals with prediabetes and found that the triglyceride-to-glucose fasting index had the best discriminatory power for predicting prediabetes in the entire population. Thus, the authors suggested that this indicator could be used as a complementary marker to assess prediabetes in the elderly [33].
The current study also targeted other factors that are proposed to have a role in predicting prediabetes. These were NLR, hsCRP, C-peptide, IL-1β, IL-13 and IL-35. The NLR, a newly identified inflammatory biomarker for DM, showed a similar significant elevation in both the prediabetes and DM groups compared to individuals with normoglycemia. These results are consistent with other reports that have shown higher NLR values in patients with prediabetes or DM and suggested that the NLR may reflect a systemic inflammatory response and could be considered a predictive and prognostic factor for diabetes and related complications [7, 8]. Regarding the predictive significance of NLR, ROC analysis of the present data revealed that NLR was a poor predictor of prediabetes or DM because the estimated AUC was in the range of 0.641–0.685. Besides, logistic regression analysis showed no significante difference of the NLR in the risk of developing prediabetes or diabetes. Therefore the increase in NLR may be related to other factors. Since neutrophils are the predominant leukocytes in peripheral blood that respond rapidly to inflammatory stimuli, their number would be expected to increase upon inflammatory responses. In addition, elevated levels of some pro-inflammatory cytokines may cause neutrophilia and lymphopenia and consequently an elevated NLR [7].
The study also showed that hsCRP and C-peptide levels were significantly elevated in the serum of prediabetes and DM patients. ROC curve analysis showed that both biomarkers were significant predictors of both conditions with AUCs of 0.829 and 0.961 for hsCRP and 0.913 and 0.892 for C-peptide in prediabetes and diabetes, respectively. Logistic regression analysis revealed that individuals in the third tertile of hsCRP and C-peptide levels (>66%) had a 23.58- and 35.33-fold increased risk of developing prediabetes, respectively, and this risk was higher in DM. Most of the published studies support these findings and confirm that hsCRP and C-peptide show up-regulated levels in patients with prediabetes or diabetes [10–13]. hsCRP is synthesized by hepatocytes in response to inflammatory stimuli and is thus considered a sensitive and systemic biomarker of inflammation, especially in diseases with low-grade chronic inflammation such as DM [9, 10]. hsCRP has also been linked to the risk of prediabetes, and patients with this condition showed significantly elevated levels of hsCRP in their serum [11]. It is interesting to note that a previous study showed that elevated levels of hsCRP were also associated with a higher risk of type 2 DM, but when categorized according to HbA1c levels, hsCRP was only positively associated with type 2 DM among those who had high levels of HbA1c (undiagnosed DM), but not low levels of HbA1c (incident DM). Therefore, elevated hsCRP levels have been suggested as byproducts of hyperglycemia, rather than directly contributing to the development of incident DM [34]. The association between hsCRP and cardiovascular events and all-cause mortality in patients with high-risk type 2 DM was also evaluated, and hsCRP was proposed as an independent risk factor for this cardiovascular complication in patients [35].
The current study revealed that C- peptide can be considered an important and relevant predictor of prediabetes and DM. C-peptide is a part of pro-insulin that is cleaved before co-secreting with insulin from pancreatic beta-cells. It is considered a useful biomarker and is superior to insulin in assessing the function of these cells because the C-peptide half-life is longer than that of insulin (20-30 vs. 3-5 minutes) and hepatic clearance is minimal compared to insulin [12]. Therefore, studies have suggested that C-peptide would be more effective in the prediction of prediabetes and DM compared to insulin [13, 36, 37]. There is also evidence to suggest that the C-peptidogenic index (C-peptide increase by glucose loading) is the most independent predictor of future DM. Further, the index was more correlated with beta-cell mass and function than the insulin-based index [38]. In addition, A positive association was found between C-peptide and the risk of developing prediabetes and DM among Chinese women with a history of gestational diabetes [13].
The final part of this study addressed the role of three cytokines in predicting prediabetes and DM. One of them is a pro-inflammatory cytokine (IL-1β), while the other two are anti-inflammatory cytokines (IL-13 and IL-35). These cytokines showed up-regulated levels in serum of prediabetes and DM patients, but the ROC curve analysis revealed that they were nearly poor predictors of prediabetes (AUC=0.672, 0.728, and 0.631, respectively), while their prognostic significance was indicated in DM (AUC=0.804, 0.868 and 0.833, respectively). Elevated levels of IL-1β were associated with an increased risk of prediabetes and DM (OR=19.49 and 49.09, respectively). Consistent with these findings, animal model studies and clinical trials have provided evidence supporting the causative role of IL-1β as an essential factor in the loss of beta-cell mass in type 2 DM. In addition, an IL-1β-mediated autoinflammatory process that leads to beta-cell death has been demonstrated [39]. It has also been found that beta-cells can produce IL-1β when stimulated with glucose and this leads to the attraction of macrophages that can contribute to increased production of IL-1β [40]. IL-1β has also been hypothesized to be a potential candidate for promoting progression from prediabetes to overt DM [16]. The present data are in good agreement with this hypothesis as IL-1β levels showed a significant increase in prediabetes compared to normoglycemia but the increase was higher in DM and the difference was significant compared to prediabetes. More supporting evidence has been recently addressed and IL-1β was considered an important predictor of new prediabetes in patients with acute pancreatitis [17]. Because of these effects of IL-1β, this cytokine has been a therapeutic target in DM and it has been indicated that anti-diabetic therapies should not only correct hyperglycemia but reduce inflammation. One advanced treatment that counteracts the pathophysiological effects of IL-1β is anakinra, and some promising results have been shown [41]. In a mouse model of gestational diabetes, increased expression of IL-1β was found in the uterus and the placenta along with elevated IL-1β serum levels compared to pregnant mice with normoglycemia. Treatment with anti-IL-1β antibodies improved glucose tolerance in mice with gestational diabetes without apparent adverse effects on the fetus [42].
As in IL-1β, the anti-inflammatory cytokine IL-13 is suggested to increase the risk of prediabetes and DM (OR=9.91 and 57.26, respectively). The results of previous studies have been conflicting regarding this issue. Serum IL-13 levels were significantly elevated in insulin-resistant patients compared to non-insulin-resistant controls [19] and in type 2 DM patients relative to controls [43]. IL-13 levels were also elevated in newly diagnosed tuberculosis patients with prediabetes [44]. In contrast, elevated levels of IL-13 have been associated with a lower risk of prediabetes, incident type 2 DM, and need for insulin therapy [20], whereas in patients with schizophrenia, serum IL-13 levels did not show significant differences between the prediabetes group and normal glucose tolerant group [45]. In patients with type 1 DM, IL-13 production showed significantly lower levels even in those with a genetic risk for disease progression, compared to healthy control individuals. In this context, IL-13 has been suggested to be an inhibitor of beta-cell destruction and is considered a key regulator of glucose metabolism [22]. Apart from these conflicting results, IL-13 appears to be an important target of research aimed at understanding the pathogenesis of DM. The increased serum level of IL-13 in current prediabetes and DM patients may be indicative of the highly complex interplay between this cytokine and its pro-inflammatory counterparts such as IL-1β. Elevated levels of IL-13 may indicate its role as a compensatory and anti-inflammatory regulatory molecule that may limit the damage caused by increased levels of pro-inflammatory cytokines [43].
The second anti-inflammatory cytokine is IL-35 and the present results indicate up-regulated levels of this cytokine in prediabetes and DM patients. ROC curve analysis revealed that IL-35 is more predictive of DM than prediabetes (AUC=0.833 and 0.631, respectively). Logistic regression analysis also confirmed that patients in the third tertile of IL-35 level (>66%) were 17.08-fold more likely to develop DM, while this increased risk was not observed in patients with prediabetes (odds ratio=3.41; p=0.096). IL-35 has not been well examined in DM and there was probably only one study that evaluated serum levels in type 2 DM patients with and without chronic periodontitis and found that both pathological conditions did not affect IL-35 levels [24]. In a rat model of DNP, intrathecal injection of IL-35 had anti-inflammatory and anti-apoptotic effects and was associated with a lower accumulation of pro-inflammatory cytokines in the spinal cord [23]. Further, low levels of IL-35 have been indicated to play a pivotal role in the development of type 1 DM and the authors recommended that the therapeutic potential of IL-35 be studied in type 1 DM [22]. Other inflammatory and autoimmune diseases are generally associated with low levels of IL-35; for instance, rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), but observations have been inconsistent [46]. In a study, the IL-35 was up-regulated at sites of inflammation in patients with RA and proposed its pro-inflammatory potential [47]. High levels of plasma IL-35 were also found in patients with active SLE who had low expression of the IL-35 receptor (gp130) on CD4+ Th cells [48]. It has been hypothesized that increased expression of IL-35 may indicate an immune-regulating association between CD4+ effector T cells and Treg cells in response to an intense inflammatory environment. Indeed, studies showed that expression of Ebi3 and p35 (IL-35 subunits) was significantly elevated in Treg cells when co-cultured with CD4+ effector T cells. This may indicate that presence of the latter cells is responsible for the conduction of IL-35 synthesis signals [46]. A recent study found that the percentage of effector memory T cells was significantly increased in type 2 DM especially in those with CVD [49]. The involvement of IL-35 in the pathogenesis of DM appears to be complex and more simultaneous evaluations, such as effector T cells, Treg cells and the IL-35 receptor, are needed.
Spearman's rank order correlation analysis between the study variables showed some significant positive correlations. Interestingly, FPG was correlated with NLR, hsCRP, C-peptide, IL-1β, IL-13, and IL-35, and similar correlations were shown with Hb1Ac except for the NLR. These results suggest a causal relationship between these variables, and hyperglycemia, as measured by FPG and Hb1Ac, may affect immune homeostasis and thus may alter the pro-inflammatory and anti-inflammatory environment in the human body. In this context, pro-inflammatory and anti-inflammatory cytokines have been proposed to be involved in DM progression, capable of interfering with insulin-responsive glucose uptake and inducing insulin resistance [50].
Regardless of the significance of these findings, some limitations should be addressed. First, the number of participants was relatively small compared to other published studies. In addition, the results of this study cannot be generalized to the rest of the Iraqi governorates because the participants were from Baghdad only. Second, only two tests (FPG and Hb1Ac) were used to define prediabetes, and these tests should be accompanied by the 2hPPG test. Third, WHO guidelines were followed to identify overweight/obese individuals, and these guidelines may have a different profile in Iraqis.
Conclusion
The prevalence of prediabetes among apparently healthy Iraqi individuals was 17%. Prediabetes was more prevalent in individuals over 40 years of age, especially those who were overweight/obese. Pro-inflammatory and anti-inflammatory cytokines were up-regulated in serum of prediabetes patients.
Acknowledgments: The authors appreciate the cooperation of the medical staff at the Primary Health Care Center in Baghdad.
Ethical Permissions: The Ethics Committee of the Iraqi Ministry of Health and Environment approved the study protocol.
Conflicts of Interests: None declared.
Authors’ Contributions: Jasim OH (First Author), Introduction Writer/Main Researcher/Statistical Analyst/
Discussion Writer (34%); Mahmood MM (Second Author), Assistant Researcher (33%); Ad'hiah AH (Third Author), Methodologist (33%)
Funding/Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Article Type: Original Research |
Subject:
Health Promotion Setting
Received: 2021/04/29 | Accepted: 2022/06/11 | Published: 2022/06/15
Received: 2021/04/29 | Accepted: 2022/06/11 | Published: 2022/06/15
References
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843. [Link] [DOI:10.1016/j.diabres.2019.107843]
2. Mohammad A, Ziyab AH, Mohammad T. Prevalence of prediabetes and undiagnosed diabetes among kuwaiti adults: A cross-sectional study. Diabetes Metab Syndr Obes Targets Ther. 2021;14:2167-76. [Link] [DOI:10.2147/DMSO.S296848]
3. Hostalek U. Global epidemiology of prediabetes - present and future perspectives. Clin Diabetes Endocrinol. 2019;5:5. [Link] [DOI:10.1186/s40842-019-0080-0]
4. Echouffo-Tcheugui JB, Selvin E. Prediabetes and what it means: the epidemiological evidence. Annu Rev Public Health. 2021;42:59-77. [Link] [DOI:10.1146/annurev-publhealth-090419-102644]
5. Kaur G, Lakshmi PVM, Rastogi A, Bhansali A, Jain S, Teerawattananon Y, et al. Diagnostic accuracy of tests for type 2 diabetes and prediabetes: a systematic review and meta-analysis. PLoS One. 2020;15(11):e0242415. [Link] [DOI:10.1371/journal.pone.0242415]
6. Vatcheva KP, Fisher-Hoch SP, Reininger BM, McCormick JB. Sex and age differences in prevalence and risk factors for prediabetes in Mexican-Americans. Diabetes Res Clin Pract. 2020;159:107950. [Link] [DOI:10.1016/j.diabres.2019.107950]
7. Duman TT, Aktas G, Atak BM, Kocak MZ, Erkus E, Savli H. Neutrophil to lymphocyte ratio as an indicative of diabetic control level in type 2 diabetes mellitus. Afr Health Sci. 2019;19:1602-6. [Link] [DOI:10.4314/ahs.v19i1.35]
8. Wang JR, Chen Z, Yang K, Yang HJ, Tao WY, Li YP, et al. Association between neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and diabetic retinopathy among diabetic patients without a related family history. Diabetol Metab Syndr. 2020;12:1-10. [Link] [DOI:10.1186/s13098-020-00562-y]
9. Kamath DY, Xavier D, Sigamani A, Pais P. High sensitivity C-reactive protein (hsCRP) & cardiovascular disease: An Indian perspective. Indian J Med Res. 2015;142(3):261. [Link] [DOI:10.4103/0971-5916.166582]
10. Shin SH, Lee YJ, Lee YA, Kim JH, Lee SY, Shin CH. High-sensitivity C-reactive protein is associated with prediabetes and adiposity in Korean youth. Metab Syndr Relat Disord. 2020;18:47-55. [Link] [DOI:10.1089/met.2019.0076]
11. Ghule A, Kamble TK, Talwar D, Kumar S, Acharya S, Wanjari A, et al. Association of serum high sensitivity c-reactive protein with pre-diabetes in rural population: a two-year cross-sectional study. Cureus. 2021;13(10):e19088. [Link] [DOI:10.7759/cureus.19088]
12. Leighton E, Sainsbury CA, Jones GC. A practical review of C-peptide testing in diabetes. Diabetes Ther. 2017;8:475-87. [Link] [DOI:10.1007/s13300-017-0265-4]
13. Yin P, Shao P, Liu H, Li W, Wang L, Wang J, et al. C-peptide levels and the risk of diabetes and pre-diabetes among Chinese women with gestational diabetes. J Diabetes Complications. 2017;31(12):1658-62. [Link] [DOI:10.1016/j.jdiacomp.2017.08.006]
14. Shi J, Fan J, Su Q, Yang Z. Cytokines and abnormal glucose and lipid metabolism. Front Endocrinol. 2019;10:703. [Link] [DOI:10.3389/fendo.2019.00703]
15. Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J. The role of interleukin-1 in general pathology. Inflamm Regen. 2019;39:12. [Link] [DOI:10.1186/s41232-019-0101-5]
16. Zhao G, Dharmadhikari G, Maedler K, Meyer-Hermann M. Possible role of Interleukin-1β in type 2 diabetes onset and implications for anti-inflammatory therapy strategies. PLoS Comput Biol. 2014;10:1003798. [Link] [DOI:10.1371/journal.pcbi.1003798]
17. Bharmal SH, Kimita W, Ko J, Petrov MS. Cytokine signature for predicting new-onset prediabetes after acute pancreatitis: A prospective longitudinal cohort study. Cytokine. 2022;150:155768. [Link] [DOI:10.1016/j.cyto.2021.155768]
18. Mao YM, Zhao CN, Leng J, Leng RX, Ye DQ, Zheng SG, et al. Interleukin-13: a promising therapeutic target for autoimmune disease. Cytokine Growth Factor Rev. 2019;45:9-23. [Link] [DOI:10.1016/j.cytogfr.2018.12.001]
19. Martínez-Reyes CP, Gómez-Arauz AY, Torres-Castro I, Manjarrez-Reyna AN, Palomera LF, Olivos-García A, et al. Serum levels of interleukin-13 increase in subjects with insulin resistance but do not correlate with markers of low-grade systemic inflammation. J Diabetes Res. 2018;2018. [Link] [DOI:10.1155/2018/7209872]
20. Brahimaj A, Ligthart S, Ghanbari M, Ikram MA, Hofman A, Franco OH, et al. Novel inflammatory markers for incident pre-diabetes and type 2 diabetes: the rotterdam study. Eur J Epidemiol. 2017;32:217-26. [Link] [DOI:10.1007/s10654-017-0236-0]
21. Song M, Ma X. The immunobiology of interleukin-35 and its regulation and gene expression. Regul Cytokine Gene Express Immun Diss. 2016;941:213-25. [Link] [DOI:10.1007/978-94-024-0921-5_10]
22. Lu J, Liu J, Li L, Lan Y, Liang Y. Cytokines in type 1 diabetes: mechanisms of action and immunotherapeutic targets. Clin Transl Immunol. 2020;9:e1122. [Link] [DOI:10.1002/cti2.1122]
23. Jiang Y, Wang J, Li H, Xia L. IL-35 alleviates inflammation progression in a rat model of diabetic neuropathic pain via inhibition of JNK signaling. J Inflamm. 2019;16:19. [Link] [DOI:10.1186/s12950-019-0217-z]
24. Maboudi A, Eghbalian-Nouzanizadeh A, Seifi H, Bahar A, Heidari M, Mohammadpour RA, et al. Serum levels of interleukin-23 and 35 in patients with and without type 2 diabetes mellitus and chronic periodontitis. Casp J Intern Med. 2019;10:295-302. [Link]
25. Galaviz KI, Narayan KMV, Lobelo F, Weber MB. Lifestyle and the prevention of type 2 diabetes: a status report. Am J Lifestyle Med. 2018;12:4-20. [Link] [DOI:10.1177/1559827615619159]
26. El-Kebbi IM, Bidikian NH, Hneiny L, Nasrallah MP. Epidemiology of type 2 diabetes in the Middle East and North Africa: Challenges and call for action. World J Diabetes. 2021;12:1401. [Link] [DOI:10.4239/wjd.v12.i9.1401]
27. Yip WCY, Sequeira IR, Plank LD, Poppitt SD. Prevalence of pre-diabetes across ethnicities: A review of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) for classification of dysglycaemia. Nutrients. 2017;9:1273. [Link] [DOI:10.3390/nu9111273]
28. Shang Y, Marseglia A, Fratiglioni L, Welmer AK, Wang R, Wang HX, et al. Natural history of prediabetes in older adults from a population-based longitudinal study. J Intern Med. 2019;286(3):326-40. [Link] [DOI:10.1111/joim.12920]
29. Tkatch R, Musich S, MacLeod S, Alsgaard K, Hawkins K, Yeh CS. Population health management for older adults: review of interventions for promoting successful aging across the health continuum. Gerontol Geriatr Med. 2016;2:2333721416667877. [Link] [DOI:10.1177/2333721416667877]
30. Batsis JA, Zagaria AB. Addressing obesity in aging patients. Med Clin North Am. 2018;102:65-85. [Link] [DOI:10.1016/j.mcna.2017.08.007]
31. Miao Z, Alvarez M, Ko A, Bhagat Y, Rahmani E, Jew B, et al. The causal effect of obesity on prediabetes and insulin resistance reveals the important role of adipose tissue in insulin resistance. PLoS Genet. 2020;16:e1009018. [Link] [DOI:10.1371/journal.pgen.1009018]
32. Gu Z, Zhu P, Wang Q, He H, Xu J, Zhang L, et al. Obesity and lipid-related parameters for predicting metabolic syndrome in Chinese elderly population. Lipids Health Dis. 2018;17:289. [Link] [DOI:10.1186/s12944-018-0927-x]
33. Ramírez-Vélez R, Pérez-Sousa MÁ, González-Ruíz K, Cano-Gutierrez CA, Schmidt-Riovalle J, Correa-Rodríguez M, et al. Obesity-and lipid-related parameters in the identification of older adults with a high risk of prediabetes according to the American diabetes association: An analysis of the. 2015 health, well-being, and aging study. Nutrients. 2019;11(11):2654. [Link] [DOI:10.3390/nu11112654]
34. Pan A, Wang Y, Yuan JM, Koh WP. High-sensitive C-reactive protein and risk of incident type 2 diabetes: A case-control study nested within the Singapore Chinese Health Study. BMC Endocr Disord. 2017;17:8. [Link] [DOI:10.1186/s12902-017-0159-5]
35. Sharif S, van der Graaf Y, Cramer MJ, Kapelle LJ, de Borst GJ, Visseren FLJ, et al. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 diabetes. Cardiovasc Diabetol. 2021;20:220. [Link] [DOI:10.1186/s12933-021-01409-0]
36. Jamiołkowska-Sztabkowska M, Głowińska-Olszewska B, Bossowski A. C-peptide and residual β-cell function in pediatric diabetes-state of the art. Pediatr Endocrinol Diabetes Metab. 2021;27(2):123-33. [Link] [DOI:10.5114/pedm.2021.107165]
37. Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30(7):803-17. [Link] [DOI:10.1111/dme.12159]
38. Jung HS. Prediction of diabetes using serum C-peptide. Endocrinol Metab. 2016;31(2):275-6. [Link] [DOI:10.3803/EnM.2016.31.2.275]
39. Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1β in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17(4):314-21. [Link] [DOI:10.1097/MED.0b013e32833bf6dc]
40. Böni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, et al. Increased interleukin (IL)-1β messenger ribonucleic acid expression in β-cells of individuals with type 2 diabetes and regulation of IL-1β in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93(10):4065-74. [Link] [DOI:10.1210/jc.2008-0396]
41. Peiró C, Lorenzo Ó, Carraro R, Sánchez-Ferrer CF. IL-1β inhibition in cardiovascular complications associated to diabetes mellitus. Front Pharmacol. 2017;8:363. [Link] [DOI:10.3389/fphar.2017.00363]
42. Schulze F, Wehner J, Kratschmar D V, Makshana V, Meier DT, Häuselmann SP, et al. Inhibition of IL-1beta improves glycaemia in a mouse model for gestational diabetes. Sci Rep. 2020;10:3035. [Link] [DOI:10.1038/s41598-020-59701-0]
43. Randeria SN, Thomson GJA, Nell TA, Roberts T, Pretorius E. Inflammatory cytokines in type 2 diabetes mellitus as facilitators of hypercoagulation and abnormal clot formation. Cardiovasc Diabetol. 2019;18:72. [Link] [DOI:10.1186/s12933-019-0870-9]
44. Hasan Z, Irfan M, Masood Q, Ahmed O, Moosajee US, Rao S, et al. Raised levels of IFN-gamma and IL-13 are associated with pre-diabetes amongst newly diagnosed patients with tuberculosis. J Pak Med Assoc. 2019;69(4):468-73. [Link]
45. Møller M, Fredholm S, Jensen ME, Wörtwein G, Larsen JR, Vilsbøll T, et al. Proinflammatory biomarkers are associated with prediabetes in patients with schizophrenia. CNS Spectr. 2020;27(3):347-54. [Link] [DOI:10.1017/S1092852920002217]
46. Su LC, Liu XY, Huang AF, Xu WD. Emerging role of IL-35 in inflammatory autoimmune diseases. Autoimmun Rev. 2018;17(7):665-73. [Link] [DOI:10.1016/j.autrev.2018.01.017]
47. Filková M, Vernerová Z, Hulejová H, Prajzlerová K, Veigl D, Pavelka K, et al. Pro-inflammatory effects of interleukin-35 in rheumatoid arthritis. Cytokine. 2015;73(1):36-43. [Link] [DOI:10.1016/j.cyto.2015.01.019]
48. Cai Z, Wong CK, Kam NW, Dong J, Jiao D, Chu M, et al. Aberrant expression of regulatory cytokine IL-35 in patients with systemic lupus erythematosus. Lupus. 2015;24:1257-66. [Link] [DOI:10.1177/0961203315585815]
49. Rattik S, Engelbertsen D, Wigren M, Ljungcrantz I, Östling G, Persson M, et al. Elevated circulating effector memory T cells but similar levels of regulatory T cells in patients with type 2 diabetes mellitus and cardiovascular disease. Diabetes Vasc Dis Res. 2019;16:270-80. [Link] [DOI:10.1177/1479164118817942]
50. Gouda W, Mageed L, Abd El Dayem SM, Ashour E, Afify M. Evaluation of pro-inflammatory and anti-inflammatory cytokines in type 1 diabetes mellitus. Bull Natl Res Cent. 2018;42:14. [Link] [DOI:10.1186/s42269-018-0016-3]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





