Volume 10, Issue 4 (2022)
Health Educ Health Promot 2022, 10(4): 687-693 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nazari M, Shafiei M, Ghahremani L. Effect of Educational Intervention through a Campaign on Health Anxiety Caused by Cancer and the Participation Rate of Middle-Aged People in Colorectal Cancer Screening Using the Health Belief Mode. Health Educ Health Promot 2022; 10 (4) :687-693
URL: http://hehp.modares.ac.ir/article-5-60090-en.html
URL: http://hehp.modares.ac.ir/article-5-60090-en.html
1- “Student Research Committee” and “Department of Health Promotion, School of Health”, Shiraz University of Medical Sciences, Shiraz, Iran
Keywords: Health Campaign [MeSH], Colorectal Cancer [MeSH], Health Belief Model [MeSH], Anxiety [MeSH]
Full-Text [PDF 1039 kb]
(1088 Downloads)
| Abstract (HTML) (877 Views)
Full-Text: (83 Views)
Introduction
Colorectal Cancer (CRC), also known as bowel cancer, accounts for 10.2% of all cancers worldwide and 9.2% of all deaths. The highest rates of prevalence and mortality have been reported in countries with very high population growth rates [1]. Approximately one million people around the world are diagnosed with CRC annually, nearly half of whom die before the fifth year after the disease onset [2]. Epidemiological studies in Iran have indicated that although the rate of CRC is still relatively low, it has increased significantly over the past three decades [3]. However, free and affordable screenings and health units are available in most areas to help diagnose the disease and educate people about healthy living and disease prevention. Screening reduces the mortality caused by CRC. Based on the current guidelines, all people over the age of 50 should be regularly screened for colon polyps and cancer [4]. CRC screening may be one of the most effective ways to prevent its progression, which can reduce the suffering, mortality, and economic costs associated with the disease [1, 2]. However, the question is why most people do not use such services and do not change their lifestyle [5].
The cause of 60% of all deaths is chronic diseases, which are also responsible for 43% of the burden of diseases in the world [6]. Chronically ill patients must deal with persistent and unpredictable diseases. These patients usually complain of anxiety and worry about their condition or the recurrence or worsening of symptoms. This is the case with cancer survivors who imagine their worries about disease recurrence as the sword of Damocles [7]. Overall, health anxiety occurs when emotions or bodily changes indicate a serious disease [8].
One of the most serious threats to public health is cancer, which is the second cause of premature death after cardiovascular disease. Screening programs implemented for the general public mean that everyone is invited to be screened. Nonetheless, cancer screening may increase cancer anxiety [9]. Selecting the health education model is the first step of planning the process in the health education course and the appropriate model starts the course by choosing the right path and maintaining the proper path. The Health Belief Model is a model used in health education [10]. Based on this model, if people believe that they are prone to such diseases as cancer (perceived sensitivity), understand the depth of this risk and the severity of its various effects on their lives (perceived severity), and perceive the recommended behaviors to reduce the severity or risk of the disease (perceived benefits), they can eliminate the factors that prevent the operation such as cost and time (perceived barriers), and have the necessary confidence in their ability to conduct the behaviors to achieve the desired result (perceived self-efficacy), and are also more likely to engage in health-promoting behaviors [11]. Langroudi et al. carried out a research on the effect of education based on the Health Belief Model on health social workers’ knowledge and attitude regarding CRC screening in Yazd and concluded that the constructs of the model can be used as a suitable framework for designing colon cancer screening training interventions to improve health-promoting behaviors [12].
Generally, increasing screening rates in a population requires interventions that are widely performed on people through media and multimedia techniques [13]. Mobilizing information refers to a set of information, communication and educational activities using a combination of diverse information channels to convey the desired messages to a specific population in a specific and limited period of time in line with the goals of the program [14]. In this context, mass media can initiate campaigns that can lead to good changes or stop negative changes in health-related behaviors in large communities [15]. In a study on the effectiveness of informing mobilization in preventing cholera amongst students, Morowati et al. concluded that holding informing mobilization at the time of health problems could result in positive changes in the target population, improve the current situation, or prevent the occurrence of further problems [14].
Considering the importance of the early diagnosis of CRC and since CRC screening is an effective and affordable way to control and prevent this disease, the current paper aimed to assess the effect of an educational intervention through a campaign on health anxiety and participation of middle-aged people (age 50-70 years) in CRC screening based on the Health Belief Model in the urban areas (Dashti and Kushkenar) of Parsian, Hormozgan Province, Iran.
Materials and Methods
The present quasi-experimental work was carried out on people aged 50 to 70 from Dashti and Kushkanar cities in Parsian, Hormozgan Province in 2021. According to Cochran's formula, the sample size was determined to be 342 people, which increased to 390 people with 15% dropout. Convenience sampling method was used to select the participants and then they were randomly divided into two intervention and control groups. At the beginning, the names of the people aged 50-70 years under the coverage of the health unit and their contact numbers were extracted using the integrated electronic health system. Then each person was assigned a code. Afterwards, the inclusion and exclusion criteria were evaluated based on the information in the individuals’ electronic health records. The inclusion criteria consisted of: 1) age 50-70 years, 2) lack of diagnosis of CRC, 3) living in the study area for more than six months, 4) not having a family history of CRC, and 5) willingness to participate in the study. The exclusion criteria included: 1) unwilling to continue cooperation in the study, 2) failure to attend all sessions, 3) not completing the questionnaires, and 4) moving to another city.
A questionnaire was used to collect data, which was completed through interviews. The questionnaire included: 1) Champion’s Health Belief Model Scale (CHBMS) and 2) Health Anxiety Questionnaire that consisted of three sections. The first section included 22 questions related to demographic characteristics. The second section consisted of 14 questions related to knowledge about CRC. Finally, the third section included 60 questions related to the constructs of the Health Belief Model and individual beliefs about CRC screening. The reliability and validity of the original version of the questionnaire was confirmed by Jacobs et al. with Cronbach's alpha of 0.60-0.78. Cronbach's alpha of the questionnaire equal to 0.78 was obtained by Bidgoli et al. (n=30) [16].
The second data collection instrument was the Health Anxiety Questionnaire. This questionnaire was first designed by Salkovskis and Warwik in 1989. This form was based on the cognitive model of health anxiety and hypochondriasis. The 18-item short form of this questionnaire was developed by Salkovskis and Warwick [17]. Nargesi et al. confirmed the construct validity and approved its reliability using Cronbach’s alpha equal to 0.75 [18].
After the pre-test for both groups, the participants of the intervention group received the intervention of ways to prevent and control CRC and anxiety caused by the disease through the campaign, which was the best way to convey information to many people during the COVID-19 pandemic. This program was implemented through posters, billboards, banners and educational messages via the SMS system for four weeks. After eight weeks, the post-test was conducted for both groups. It is worth noting that the participants ensured the confidentiality of the information and sent a written consent form. The Ethics Committee of Shiraz University of Medical Sciences approved the study (IR.SUMS.REC.1399.1207).
Data analysis was done using descriptive statistics and inferential statistics in SPSS 26 software. To investigate the effect of training on the scores of the participants in the research, analysis of covariance was used to control the effect of the scores before training and to compare the effect of training in two groups.
Findings
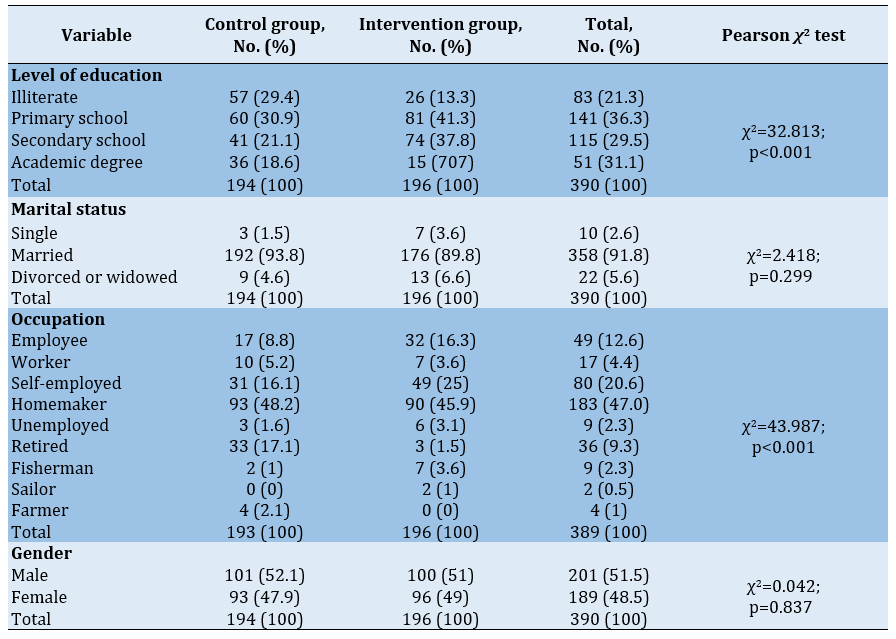
In the pre-test, there was no significant difference between the intervention and control groups in terms of demographic variables (gender, age, and marital status, and economic status). However, the intervention and control groups had a significant difference in terms of occupation; the majority of the participants (47.0%) were homemaker. Additionally, 86.9% of the participants had a diploma and 13.1% had a academic degree (Table 1).
Table 1) The frequency distributions of demographic variables
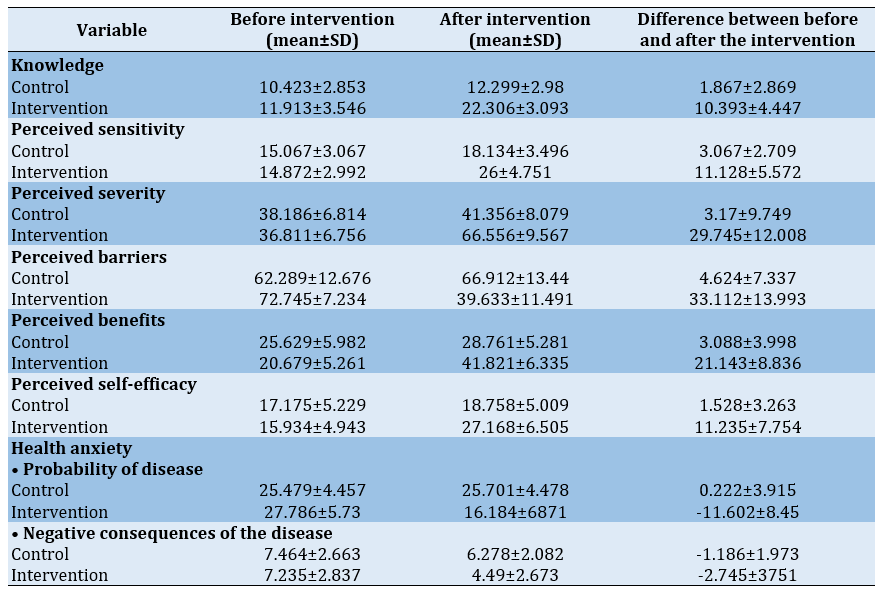
The mean scores of the constructs of the Health Belief Model and the health anxiety questionnaire in the control and intervention groups, before and after the intervention, are presented in Table 2.
Table 2) The mean scores of the Health Belief Model constructs and the Health Anxiety Questionnaire before and after the intervention
The control and intervention groups were significantly different in terms of participation in CRC screening before and after the intervention.
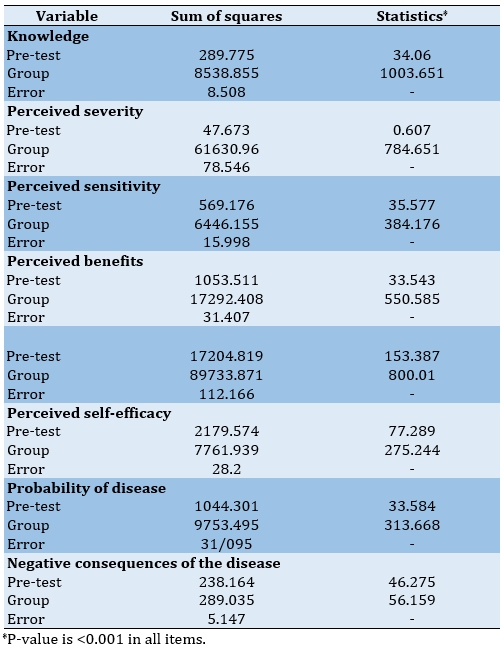
In the pre-test phase, the mean scores of knowledge, perceived severity, perceived sensitivity, perceived barriers, perceived benefits, perceived self-efficacy, as well as the scores of the probability of contracting the disease and the negative consequences of contracting the disease were significantly different between the two control and intervention groups. By controlling the effect of pre-training scores, the p-value for the group factor was less than 0.001 for all hypotheses. Therefore, it was confirmed that training had a positive effect on increasing the scores of the constructs (Table 3).
Table 3) The results of the analysis of covariance to compare the post-test scores of two groups
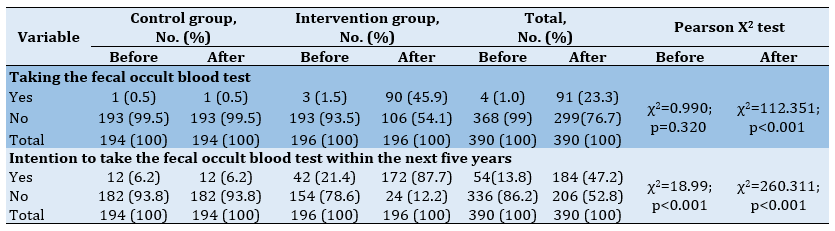
Before the intervention, only 1.8% of the participants had heard or read about colon cancer screening. In this regard, there was no significant difference between the two groups (p=0.713). After the intervention, 94.9% of the participants in the intervention group had heard or read about colon cancer screening and there was a significant difference between the two groups (p<0.001). Most of the participants received 47.2% of their information from health care personnel, and the two groups were significantly different in this regard. Besides, the majority of the participants (55.6%) stated their reason for doing the screening test was the physician's recommendation, and the two groups had a significant difference in this respect (Table 4).
Table 4) Frequency and percentage of participation in screening in the control and intervention groups before and after the intervention
Discussion
There was a significant difference between the control and intervention groups in terms of the mean score of knowledge after the intervention. The results of the univariate analysis of covariance on the post-test scores in the intervention and control groups show that educational programs have increased the knowledge score in the intervention group compared to the control group. Therefore, education had a positive effect on increasing knowledge scores. These findings were in line with the findings of Gimeno-García et al. [19], Maxwell et al. [20], Mojica et al. [21], and Langroudi et al. [12].
The findings indicated that there is a significant difference between the groups in terms of perceived sensitivity score after the intervention. Accordingly, the results of univariate analysis of covariance indicated that the increase in perceived sensitivity scores was more prominent in the intervention group than in the control group. Hence, training had a positive effect on increasing perceived sensitivity scores. These findings are in line with the findings of Kouhpayeh et al. [22], Jeihooni et al. [23], Alidosti et al. [24], and Roozitalab et al. [25].
The findings of the present study showed that there is a significant difference between the two groups in terms of the average score of perceived severity after the intervention. The results of univariable analysis of covariance on the post-test scores in the intervention and control groups indicated that the educational programs increase the perceived severity score in the intervention group compared to the control group. Thus, education had a positive impact on increasing the perceived severity scores. These findings are in agreement with the findings of Farmanfarma et al. [26], Kolutek et al. [27], and Wang et al. [28].
It was also indicated that the two groups were significantly different in terms of the mean score of perceived barriers following the intervention. The results of the univariate analysis of covariance on the post-test scores in the intervention and control groups show that educational programs have decreased the perceived barriers score in the intervention group compared to the control group. Therefore, education had a positive impact on reducing the score of perceived barriers. These results were similar to those obtained by Langroudi et al. [12] and Gimeno-García et al. [19], and inconsistent with the research performed by Pirzadeh et al. [29].
The findings of the present study revealed that the two groups were significantly different in terms of the mean score of perceived benefits following the intervention. In the same way, the results of the analysis of covariance showed that the mean score of perceived benefits in the intervention group was significantly higher than the control group. Therefore, education had a positive effect on the score of perceived benefits. These findings are in line with the findings of Grace X Ma et al. [30], Alidosti et al. [24], Rakhshandehrou et al. [31], and Abood et al. [32], but not with the the findings of of Hay et al. [33].
The findings indicated that the two study groups were significantly different concerning the mean score of perceived self-efficacy. The results of the analysis of covariance indicated that the mean score in the intervention group was significantly higher than the control group. Thus, education had a positive impact on increasing the perceived self-efficacy scores, which was consistent with the findings of Langroudi et al. [12], Broun et al. [34], Gholampour et al. [35], Khani Jeyhooni et al. [23], and Wong et al. [36].
The most important action guide for doing the screening test was the physician’s recommendation in the intervention group (56.6%), which was consistent with the findings of Javadzadeh et al. [37], Shokar et al. [38], Cyr et al. [39], Khani Jeyhooni et al. [23], and Moghimi-Dehkordi et al. [40].
The results of the analysis of covariance showed that the intervention and control groups have a significant difference in terms of the mean score of probability of disease following the intervention. In the same way, this mean score in the intervention group was lower compared to the control group. Therefore, education positively affect on reducing the score of the disease risk. Literature review revealed no similar studies to compare the results.
The findings of the study showed that the two groups were significantly different in terms of the negative consequences of the disease after the intervention. The decrease in the score of negative consequences of the disease was more in the intervention group than in the control group. However, no similar studies were found to compare the findings.
Comparison of the two groups in terms of participation in CRC screening after the intervention showed that the percentage of participants in the intervention group undergoing fecal occult blood testing in the past year (45%) increased. There was also a significant difference between the control and intervention groups in this regard, which was in line with the results of Moattar et al. [41], Khani Jeihooni et al. [23], and Bae et al. [42].
One of the strengths of this research was its comparative design, which increased the reliability of the work and achieved more accurate results. Another strength of the research was the use of all the constructs of the Health Belief Model, which led to more comprehensive findings.
One of the limitations of the study was the impossibility of holding a face-to-face meeting for the intervention group due to the COVID-19 pandemic. Furthermore, considering the age of the participants, access to social networks was difficult in some cases.
Since middle-aged people are among the high-risk groups, the implementation of educational interventions using the Health Belief Model should be considered as an efficient strategy to increase the participation of these people in cancer screening.
Conclusion
Education using the Health Belief Model is successful in cancer screening and health anxiety caused by this disease. Implementation of an educational program for people aged 50 to 70 years leads to increased participation in CRC screening and reduced health anxiety.
The results of this study can be widely used to improve the activities of health care professionals including doctors, nurses and health care providers. By using the results of this study in developing appropriate educational programs at the community level, it is possible to increase people's participation in colorectal cancer screening programs. The findings of this study suggest that managers of health care centers and educational institutions take appropriate action regarding the necessity of screening by improving the health status of the society.
Acknowledgements: The authors hereby express their gratitude to the professors of the Department of Health Education and Health Promotion of Shiraz University of Medical Sciences who sincerely helped in conducting this study. They also appreciate all the colleagues in Dashti and Kushkanar Comprehensive Health Centers and all the people who took steps to better understand the health issues in the population. We are also grateful to Ms. A. Keivanshekouh at the Research Consulting Center (RCC) of Shiraz University of Medical Sciences for the proofreading.
Ethical Permission: This project has been approved by the Student Research Committee of Shiraz University of Medical Sciences (research code: 22313, ethical code: IR.SUMS.REC.1399.1207). There was no bias in using the findings of the articles and all the positive and negative information was used in writing this article.
Conflict of Interests: There was no conflict of interests.
Authors’ Contribution: Nazari M. (First author), Introduction author/Methodologist/Original researcher/Discussion author (50%); Shafiei M.H. (Second author), Introduction author/Original researcher/ Statistical analyst/Discussion author (30%); Ghahremani L. (Third author), Methodologist/Assistant/ Statistical analyst (20%)
Funding: This project is a student thesis with the code 22313, which has been approved by the student research committee of Shiraz University of Medical Sciences, and the financial resources of the project are under the supervision of Shiraz University of Medical Sciences.
Colorectal Cancer (CRC), also known as bowel cancer, accounts for 10.2% of all cancers worldwide and 9.2% of all deaths. The highest rates of prevalence and mortality have been reported in countries with very high population growth rates [1]. Approximately one million people around the world are diagnosed with CRC annually, nearly half of whom die before the fifth year after the disease onset [2]. Epidemiological studies in Iran have indicated that although the rate of CRC is still relatively low, it has increased significantly over the past three decades [3]. However, free and affordable screenings and health units are available in most areas to help diagnose the disease and educate people about healthy living and disease prevention. Screening reduces the mortality caused by CRC. Based on the current guidelines, all people over the age of 50 should be regularly screened for colon polyps and cancer [4]. CRC screening may be one of the most effective ways to prevent its progression, which can reduce the suffering, mortality, and economic costs associated with the disease [1, 2]. However, the question is why most people do not use such services and do not change their lifestyle [5].
The cause of 60% of all deaths is chronic diseases, which are also responsible for 43% of the burden of diseases in the world [6]. Chronically ill patients must deal with persistent and unpredictable diseases. These patients usually complain of anxiety and worry about their condition or the recurrence or worsening of symptoms. This is the case with cancer survivors who imagine their worries about disease recurrence as the sword of Damocles [7]. Overall, health anxiety occurs when emotions or bodily changes indicate a serious disease [8].
One of the most serious threats to public health is cancer, which is the second cause of premature death after cardiovascular disease. Screening programs implemented for the general public mean that everyone is invited to be screened. Nonetheless, cancer screening may increase cancer anxiety [9]. Selecting the health education model is the first step of planning the process in the health education course and the appropriate model starts the course by choosing the right path and maintaining the proper path. The Health Belief Model is a model used in health education [10]. Based on this model, if people believe that they are prone to such diseases as cancer (perceived sensitivity), understand the depth of this risk and the severity of its various effects on their lives (perceived severity), and perceive the recommended behaviors to reduce the severity or risk of the disease (perceived benefits), they can eliminate the factors that prevent the operation such as cost and time (perceived barriers), and have the necessary confidence in their ability to conduct the behaviors to achieve the desired result (perceived self-efficacy), and are also more likely to engage in health-promoting behaviors [11]. Langroudi et al. carried out a research on the effect of education based on the Health Belief Model on health social workers’ knowledge and attitude regarding CRC screening in Yazd and concluded that the constructs of the model can be used as a suitable framework for designing colon cancer screening training interventions to improve health-promoting behaviors [12].
Generally, increasing screening rates in a population requires interventions that are widely performed on people through media and multimedia techniques [13]. Mobilizing information refers to a set of information, communication and educational activities using a combination of diverse information channels to convey the desired messages to a specific population in a specific and limited period of time in line with the goals of the program [14]. In this context, mass media can initiate campaigns that can lead to good changes or stop negative changes in health-related behaviors in large communities [15]. In a study on the effectiveness of informing mobilization in preventing cholera amongst students, Morowati et al. concluded that holding informing mobilization at the time of health problems could result in positive changes in the target population, improve the current situation, or prevent the occurrence of further problems [14].
Considering the importance of the early diagnosis of CRC and since CRC screening is an effective and affordable way to control and prevent this disease, the current paper aimed to assess the effect of an educational intervention through a campaign on health anxiety and participation of middle-aged people (age 50-70 years) in CRC screening based on the Health Belief Model in the urban areas (Dashti and Kushkenar) of Parsian, Hormozgan Province, Iran.
Materials and Methods
The present quasi-experimental work was carried out on people aged 50 to 70 from Dashti and Kushkanar cities in Parsian, Hormozgan Province in 2021. According to Cochran's formula, the sample size was determined to be 342 people, which increased to 390 people with 15% dropout. Convenience sampling method was used to select the participants and then they were randomly divided into two intervention and control groups. At the beginning, the names of the people aged 50-70 years under the coverage of the health unit and their contact numbers were extracted using the integrated electronic health system. Then each person was assigned a code. Afterwards, the inclusion and exclusion criteria were evaluated based on the information in the individuals’ electronic health records. The inclusion criteria consisted of: 1) age 50-70 years, 2) lack of diagnosis of CRC, 3) living in the study area for more than six months, 4) not having a family history of CRC, and 5) willingness to participate in the study. The exclusion criteria included: 1) unwilling to continue cooperation in the study, 2) failure to attend all sessions, 3) not completing the questionnaires, and 4) moving to another city.
A questionnaire was used to collect data, which was completed through interviews. The questionnaire included: 1) Champion’s Health Belief Model Scale (CHBMS) and 2) Health Anxiety Questionnaire that consisted of three sections. The first section included 22 questions related to demographic characteristics. The second section consisted of 14 questions related to knowledge about CRC. Finally, the third section included 60 questions related to the constructs of the Health Belief Model and individual beliefs about CRC screening. The reliability and validity of the original version of the questionnaire was confirmed by Jacobs et al. with Cronbach's alpha of 0.60-0.78. Cronbach's alpha of the questionnaire equal to 0.78 was obtained by Bidgoli et al. (n=30) [16].
The second data collection instrument was the Health Anxiety Questionnaire. This questionnaire was first designed by Salkovskis and Warwik in 1989. This form was based on the cognitive model of health anxiety and hypochondriasis. The 18-item short form of this questionnaire was developed by Salkovskis and Warwick [17]. Nargesi et al. confirmed the construct validity and approved its reliability using Cronbach’s alpha equal to 0.75 [18].
After the pre-test for both groups, the participants of the intervention group received the intervention of ways to prevent and control CRC and anxiety caused by the disease through the campaign, which was the best way to convey information to many people during the COVID-19 pandemic. This program was implemented through posters, billboards, banners and educational messages via the SMS system for four weeks. After eight weeks, the post-test was conducted for both groups. It is worth noting that the participants ensured the confidentiality of the information and sent a written consent form. The Ethics Committee of Shiraz University of Medical Sciences approved the study (IR.SUMS.REC.1399.1207).
Data analysis was done using descriptive statistics and inferential statistics in SPSS 26 software. To investigate the effect of training on the scores of the participants in the research, analysis of covariance was used to control the effect of the scores before training and to compare the effect of training in two groups.
Findings
In the pre-test, there was no significant difference between the intervention and control groups in terms of demographic variables (gender, age, and marital status, and economic status). However, the intervention and control groups had a significant difference in terms of occupation; the majority of the participants (47.0%) were homemaker. Additionally, 86.9% of the participants had a diploma and 13.1% had a academic degree (Table 1).
Table 1) The frequency distributions of demographic variables

The mean scores of the constructs of the Health Belief Model and the health anxiety questionnaire in the control and intervention groups, before and after the intervention, are presented in Table 2.
Table 2) The mean scores of the Health Belief Model constructs and the Health Anxiety Questionnaire before and after the intervention

The control and intervention groups were significantly different in terms of participation in CRC screening before and after the intervention.
In the pre-test phase, the mean scores of knowledge, perceived severity, perceived sensitivity, perceived barriers, perceived benefits, perceived self-efficacy, as well as the scores of the probability of contracting the disease and the negative consequences of contracting the disease were significantly different between the two control and intervention groups. By controlling the effect of pre-training scores, the p-value for the group factor was less than 0.001 for all hypotheses. Therefore, it was confirmed that training had a positive effect on increasing the scores of the constructs (Table 3).
Table 3) The results of the analysis of covariance to compare the post-test scores of two groups

Before the intervention, only 1.8% of the participants had heard or read about colon cancer screening. In this regard, there was no significant difference between the two groups (p=0.713). After the intervention, 94.9% of the participants in the intervention group had heard or read about colon cancer screening and there was a significant difference between the two groups (p<0.001). Most of the participants received 47.2% of their information from health care personnel, and the two groups were significantly different in this regard. Besides, the majority of the participants (55.6%) stated their reason for doing the screening test was the physician's recommendation, and the two groups had a significant difference in this respect (Table 4).
Table 4) Frequency and percentage of participation in screening in the control and intervention groups before and after the intervention

Discussion
There was a significant difference between the control and intervention groups in terms of the mean score of knowledge after the intervention. The results of the univariate analysis of covariance on the post-test scores in the intervention and control groups show that educational programs have increased the knowledge score in the intervention group compared to the control group. Therefore, education had a positive effect on increasing knowledge scores. These findings were in line with the findings of Gimeno-García et al. [19], Maxwell et al. [20], Mojica et al. [21], and Langroudi et al. [12].
The findings indicated that there is a significant difference between the groups in terms of perceived sensitivity score after the intervention. Accordingly, the results of univariate analysis of covariance indicated that the increase in perceived sensitivity scores was more prominent in the intervention group than in the control group. Hence, training had a positive effect on increasing perceived sensitivity scores. These findings are in line with the findings of Kouhpayeh et al. [22], Jeihooni et al. [23], Alidosti et al. [24], and Roozitalab et al. [25].
The findings of the present study showed that there is a significant difference between the two groups in terms of the average score of perceived severity after the intervention. The results of univariable analysis of covariance on the post-test scores in the intervention and control groups indicated that the educational programs increase the perceived severity score in the intervention group compared to the control group. Thus, education had a positive impact on increasing the perceived severity scores. These findings are in agreement with the findings of Farmanfarma et al. [26], Kolutek et al. [27], and Wang et al. [28].
It was also indicated that the two groups were significantly different in terms of the mean score of perceived barriers following the intervention. The results of the univariate analysis of covariance on the post-test scores in the intervention and control groups show that educational programs have decreased the perceived barriers score in the intervention group compared to the control group. Therefore, education had a positive impact on reducing the score of perceived barriers. These results were similar to those obtained by Langroudi et al. [12] and Gimeno-García et al. [19], and inconsistent with the research performed by Pirzadeh et al. [29].
The findings of the present study revealed that the two groups were significantly different in terms of the mean score of perceived benefits following the intervention. In the same way, the results of the analysis of covariance showed that the mean score of perceived benefits in the intervention group was significantly higher than the control group. Therefore, education had a positive effect on the score of perceived benefits. These findings are in line with the findings of Grace X Ma et al. [30], Alidosti et al. [24], Rakhshandehrou et al. [31], and Abood et al. [32], but not with the the findings of of Hay et al. [33].
The findings indicated that the two study groups were significantly different concerning the mean score of perceived self-efficacy. The results of the analysis of covariance indicated that the mean score in the intervention group was significantly higher than the control group. Thus, education had a positive impact on increasing the perceived self-efficacy scores, which was consistent with the findings of Langroudi et al. [12], Broun et al. [34], Gholampour et al. [35], Khani Jeyhooni et al. [23], and Wong et al. [36].
The most important action guide for doing the screening test was the physician’s recommendation in the intervention group (56.6%), which was consistent with the findings of Javadzadeh et al. [37], Shokar et al. [38], Cyr et al. [39], Khani Jeyhooni et al. [23], and Moghimi-Dehkordi et al. [40].
The results of the analysis of covariance showed that the intervention and control groups have a significant difference in terms of the mean score of probability of disease following the intervention. In the same way, this mean score in the intervention group was lower compared to the control group. Therefore, education positively affect on reducing the score of the disease risk. Literature review revealed no similar studies to compare the results.
The findings of the study showed that the two groups were significantly different in terms of the negative consequences of the disease after the intervention. The decrease in the score of negative consequences of the disease was more in the intervention group than in the control group. However, no similar studies were found to compare the findings.
Comparison of the two groups in terms of participation in CRC screening after the intervention showed that the percentage of participants in the intervention group undergoing fecal occult blood testing in the past year (45%) increased. There was also a significant difference between the control and intervention groups in this regard, which was in line with the results of Moattar et al. [41], Khani Jeihooni et al. [23], and Bae et al. [42].
One of the strengths of this research was its comparative design, which increased the reliability of the work and achieved more accurate results. Another strength of the research was the use of all the constructs of the Health Belief Model, which led to more comprehensive findings.
One of the limitations of the study was the impossibility of holding a face-to-face meeting for the intervention group due to the COVID-19 pandemic. Furthermore, considering the age of the participants, access to social networks was difficult in some cases.
Since middle-aged people are among the high-risk groups, the implementation of educational interventions using the Health Belief Model should be considered as an efficient strategy to increase the participation of these people in cancer screening.
Conclusion
Education using the Health Belief Model is successful in cancer screening and health anxiety caused by this disease. Implementation of an educational program for people aged 50 to 70 years leads to increased participation in CRC screening and reduced health anxiety.
The results of this study can be widely used to improve the activities of health care professionals including doctors, nurses and health care providers. By using the results of this study in developing appropriate educational programs at the community level, it is possible to increase people's participation in colorectal cancer screening programs. The findings of this study suggest that managers of health care centers and educational institutions take appropriate action regarding the necessity of screening by improving the health status of the society.
Acknowledgements: The authors hereby express their gratitude to the professors of the Department of Health Education and Health Promotion of Shiraz University of Medical Sciences who sincerely helped in conducting this study. They also appreciate all the colleagues in Dashti and Kushkanar Comprehensive Health Centers and all the people who took steps to better understand the health issues in the population. We are also grateful to Ms. A. Keivanshekouh at the Research Consulting Center (RCC) of Shiraz University of Medical Sciences for the proofreading.
Ethical Permission: This project has been approved by the Student Research Committee of Shiraz University of Medical Sciences (research code: 22313, ethical code: IR.SUMS.REC.1399.1207). There was no bias in using the findings of the articles and all the positive and negative information was used in writing this article.
Conflict of Interests: There was no conflict of interests.
Authors’ Contribution: Nazari M. (First author), Introduction author/Methodologist/Original researcher/Discussion author (50%); Shafiei M.H. (Second author), Introduction author/Original researcher/ Statistical analyst/Discussion author (30%); Ghahremani L. (Third author), Methodologist/Assistant/ Statistical analyst (20%)
Funding: This project is a student thesis with the code 22313, which has been approved by the student research committee of Shiraz University of Medical Sciences, and the financial resources of the project are under the supervision of Shiraz University of Medical Sciences.
Article Type: Original Research |
Subject:
Health Education and Health Behavior
Received: 2022/03/6 | Accepted: 2022/07/27 | Published: 2022/09/20
Received: 2022/03/6 | Accepted: 2022/07/27 | Published: 2022/09/20
References
1. D'Onise K, Iacobini ET, Canuto KJ. Colorectal cancer screening using feacal occult blood tests for Indigenous adults: A systematic literature review of barriers, enablers and implemented strategies. Prev Med. 2020;134:106018. [Link] [DOI:10.1016/j.ypmed.2020.106018]
2. Newton KF, Newman W, Hill J. Review of biomarkers in colorectal cancer. Colorectal Dis. 2012;14(1):3-17. [Link] [DOI:10.1111/j.1463-1318.2010.02439.x]
3. Malekzadeh R, Bishehsari F, Mahdavinia M, Ansari R. Epidemiology and molecular genetics of colorectal cancer in iran: a review. Arch Iran Med. 2009;12(2):161-9. [Link]
4. Beeker C, Kraft JM, Southwell BG, Jorgensen CM. Colorectal cancer screening in older men and women: qualitative research findings and implications for intervention. J Community Health. 2000;25(3):263-78. [Link] [DOI:10.1023/A:1005104406934]
5. Rouzitalab M, Moatari M, Gholamzadeh S, Saberi Firouzi M, Zare N. The effect of health belief on participation of the official administrative personnel in colorectal cancer screening programs in Shiraz University of Medical Sciences: 2004. Govaresh J. 2008;13(1):19-24. [Persian] [Link]
6. World Health Organization. Global status report on noncommunicable diseases 2014 [Internet]. Geneva: World Health Organization; 2014 [cited 2022 Apr 30]. Available from: https://apps.who.int/iris/handle/10665/148114. [Link]
7. Lebel S, Mutsaers B, Tomei C, Leclair CS, Jones G, Petricone-Westwood D, et al. Health anxiety and illness-related fears across diverse chronic illnesses: A systematic review on conceptualization, measurement, prevalence, course, and correlates. PloS One. 2020;15(7):e0234124. [Link] [DOI:10.1371/journal.pone.0234124]
8. Asmundson GJ, Taylor S, Carleton RN, Weeks JW, Hadjstavropoulos HD. Should health anxiety be carved at the joint? A look at the health anxiety construct using factor mixture modeling in a non-clinical sample. J Anxiety Disord. 2012;26(1):246-51. [Link] [DOI:10.1016/j.janxdis.2011.11.009]
9. Miles A, Wardle J. Adverse psychological outcomes in colorectal cancer screening: does health anxiety play a role? Behav Res Ther. 2006;44(8):1117-27. [Link] [DOI:10.1016/j.brat.2005.08.011]
10. Guvenc G, Akyuz A, Açikel CH. Health belief model scale for cervical cancer and Pap smear test: psychometric testing. J Adv Nurs. 2011;67(2):428-37. [Link] [DOI:10.1111/j.1365-2648.2010.05450.x]
11. Namdar A, Bigizadeh S, Naghizadeh MM. Measuring Health Belief Model components in adopting preventive behaviors of cervical cancer. J Fasa Univ Med Sci. 2012;2(1):34-44. [Persian] [Link]
12. Alavi Langroodi S, Fallahzadeh H, Mostafavi F. The effect of education based on health belief model on knowledge and attitude of health care workers towards colon cancer screening in Yazd. J Health Syst Res. 2019;15(3):177-83. [Persian] [Link] [DOI:10.32592/hsr.2020.15.3.109]
13. Durkin SJ, Broun K, Spittal MJ, Wakefield MA. Impact of a mass media campaign on participation rates in a National Bowel Cancer Screening Program: a field experiment. BMJ Open. 2019;9(1):e024267. [Link] [DOI:10.1136/bmjopen-2018-024267]
14. Morowatisharifabad M, Bahmani A, Ahmadian F, Vatankhah M, Gharib A. Assessing the effectiveness of cholera prevention campaign in students. J Nurs Educ. 2015;3(4):11-8. [Persian] [Link]
15. Wakefield MA, Loken B, Hornik RC. Use of mass media campaigns to change health behaviour. Lancet. 2010;376(9748):1261-71. [Link] [DOI:10.1016/S0140-6736(10)60809-4]
16. Shouri Bidgoli AR, Taheri Kharame Z, Asayesh H, Sharififard F, Sheydaiyan Arani M, Hajaligol A, et al. A study of knowledge, attitude, and practiceoncolorectal cancer screening among individuals older than 50 years based on health belief model. Qom Univ Med Sci J. 2015;9(1):59-65. [Persian] [Link]
17. Salkovskis PM, Rimes KA, Warwick HMC, Clark D. The Health Anxiety Inventory: development and validation of scales for the measurement of health anxiety and hypochondriasis. Psychol Med. 2002;32(5):843-53. [Link] [DOI:10.1017/S0033291702005822]
18. Nargesi F, Izadi F, Kariminejad K, Rezaii Sharif Ali. The investigation of the reliability and validity of persian version of health anxiety questionnaire in students of Lorestan University of Medical Sciences. Q Educ Measurement. 2017;7(27):147-60. [Persian] [Link]
19. Gimeno-García A, Quintero E, Nicolás-Pérez D, Jiménez-Sosa A. Public awareness of colorectal cancer and screening in a Spanish population. Public Health. 2011;125(9):609-15. [Link] [DOI:10.1016/j.puhe.2011.03.014]
20. Maxwell AE, Bastani R, Danao LL, Antonio C, Garcia GM, Crespi CM. Results of a community-based randomized trial to increase colorectal cancer screening among Filipino Americans. Am J Public Health. 2010;100(11):2228-34. [Link] [DOI:10.2105/AJPH.2009.176230]
21. Mojica CM, Morales-Campos DY, Carmona CM, Ouyang Y, Liang Y. Breast, cervical, and colorectal cancer education and navigation: results of a community health worker intervention. Health Promot Pract. 2016;17(3):353-63. [Link] [DOI:10.1177/1524839915603362]
22. Kouhpayeh A, Jeihooni AK, Kashfi SH, Bahmandoost M. Effect of an educational intervention based on the model of health beliefs in self-medication of Iranian mothers. Invest Educ Enferm. 2017;35(1):59-68. [Link] [DOI:10.17533/udea.iee.v35n1a07]
23. Khani Jeihooni A, Askari A, Kashfi SM, Khiyali Z, Kashfi SH, Safari O, et al. Application of health belief model in prevention of osteoporosis among primary school girl students. Int J Pediatr. 2017;5(11):6017-29. [Link]
24. Alidosti M, Sharifirad G, Hemate Z, Delaram M, Najimi A, Tavassoli E. The effect of education based on health belief model of nutritional behaviors associated with gastric cancer in housewives of Isfahan city. Daneshvar Med. 2011;18(94):1-11. [Persian] [Link]
25. Roozitalab M, Moatari M, Gholamzadeh S, SaberiFiroozi M, Zare N. The effect of health belief on participation of the official administrative personnel in colorectal cancer screening programs in Shiraz University of Medical Sciences: 2004. Govaresh. 2012;13(1):19-24. [Persian] [Link]
26. Kalan Farmanfarma K, Zareban I, Jalili Z, Shahrakipour M. Effectiveness of education based on the health belief model on performing pre-ventive measures for breast cancer among female teachers in Zahedan. J Educ Community Health. 2014;1(1):11-8. [Link] [DOI:10.20286/jech-010111]
27. Kolutek R, Avci IA, Sevig U. 84 the effects of scheduled observation at home on health beliefs related to breast and cervical cancer screening and attitudes of married women. Eur J Oncol Nurs. 2014;18(1):S25. [Link] [DOI:10.1016/S1462-3889(14)70103-6]
28. Wang W-L, Hsu S-D, Wang J-H, Huang L-C, Hsu W-L. Survey of breast cancer mammography screening behaviors in Eastern Taiwan based on a health belief model. Kaohsiung J Med Sci. 2014;30(8):422-7. [Link] [DOI:10.1016/j.kjms.2014.04.007]
29. Pirzadeh A, Mazaheri MA. The effect of education on women's practice based on the health belief model about pap smear test. Int J Prev Med. 2012;3(8):585-90. [Link]
30. Ma GX, Shive S, Tan Y, Gao W, Rhee J, Park M, et al. Community-based colorectal cancer intervention in underserved Korean Americans. Cancer Epidemiol. 2009;33(5):381-6. [Link] [DOI:10.1016/j.canep.2009.10.001]
31. Rakhshanderou S, Maghsoudloo M, Safari-Moradabadi A, Ghaffari M. Theoretically designed interventions for colorectal cancer prevention: a case of the health belief model. BMC Med Educ. 2020;20(1):1-8. [Link] [DOI:10.1186/s12909-020-02192-4]
32. Abood DA, Black DR, Feral D. Nutrition education worksite intervention for university staff: application of the health belief model. J Nutr Educ Behav. 2003;35(5):260-7. [Link] [DOI:10.1016/S1499-4046(06)60057-2]
33. Hay JL, Ford JS, Klein D, Primavera LH, Buckley TR, Stein TR, et al. Adherence to colorectal cancer screening in mammography-adherent older women. J Behav Med. 2003;26(6):553-76. [Link] [DOI:10.1023/A:1026253802962]
34. Braun KL, Fong M, Kaanoi ME, Kamaka ML, Gotay CC. Testing a culturally appropriate, theory-based intervention to improve colorectal cancer screening among Native Hawaiians. Prev Med. 2005;40(6):619-27. [Link] [DOI:10.1016/j.ypmed.2004.09.005]
35. Gholampour Y, Jaderipour A, Jeihooni AK, Kashfi SM, Harsini PA. The effect of educational intervention based on health belief model and social support on the rate of participation of individuals in performing fecal occult blood test for colorectal cancer screening. Asian Pac J Cancer Prev. 2018;19(10):2777-87. [Link]
36. Wong RK, Wong ML, Chan YH, Feng Z, Wai CT, Yeoh KG. Gender differences in predictors of colorectal cancer screening uptake: a national cross sectional study based on the health belief model. BMC Public Health. 2013;13(1):677. [Linkvv] [DOI:10.1186/1471-2458-13-677]
37. Javadzadeh Sha, Mostafavi F, Emami Smh, Hassanzadeh A, Sharifirad G. Factors associated with the fecal occult blood testing for colorectal cancer screening based on health belief model structures in moderate risk individuals, Isfahan, Iran. Health System Research. J Educ Health Promot. 2012;1:18. [Link] [DOI:10.4103/2277-9531.99218]
38. Shokar NK, Carlson CA, Weller SC. Factors associated with racial/ethnic differences in colorectal cancer screening. The Journal of the American Board of Family Medicine. 2008;21(5):414-26. [Link] [DOI:10.3122/jabfm.2008.05.070266]
39. Cyr A, Dunnagan TA, Haynes G. Efficacy of the health belief model for predicting intention to pursue genetic testing for colorectal cancer. J Genet Couns. 2010;19(2):174-86. [Link] [DOI:10.1007/s10897-009-9271-7]
40. Moghimi-Dehkordi B, Safaee A. An overview of colorectal cancer survival rates and prognosis in Asia. World J Gastrointest Oncol. 2012;4(4):71-5. [Link] [DOI:10.4251/wjgo.v4.i4.71]
41. Moattar M, Roozitalab M, Gholamzadeh S, Firoozi M, Zare N. Practical application of health belief model to enhance the uptake of colorectal cancer screening. J Community Med Health Educ. 2014;4(4):297. [Link] [DOI:10.4172/2161-0711.1000297]
42. Bae N, Park S, Lim S. Factors associated with adherence to fecal occult blood testing for colorectal cancer screening among adults in the Republic of Korea. Eur J Oncol Nurs. 2014;18(1):72-7. [Link] [DOI:10.1016/j.ejon.2013.09.001]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






