Volume 13, Issue 2 (2025)
Health Educ Health Promot 2025, 13(2): 213-220 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sharma S, Tilak Francis T. Post-Isometric Relaxation for Management of Hemophilic Arthropathy. Health Educ Health Promot 2025; 13 (2) :213-220
URL: http://hehp.modares.ac.ir/article-5-78818-en.html
URL: http://hehp.modares.ac.ir/article-5-78818-en.html
1- Department of Physiotherapy, Krupanidhi College of Physiotherapy, Rajiv Gandhi University of Health Sciences, Bangalore, India
Keywords: Hemophilia A [MeSH], Joint Diseases [MeSH], Physical Therapy Modalities [MeSH], Muscle Relaxation [MeSH], Range of Motion, Articular [MeSH], Functional Status [MeSH]
Full-Text [PDF 667 kb]
(265 Downloads)
| Abstract (HTML) (1167 Views)
Full-Text: (88 Views)
Introduction
Hemophilia is an X-linked inherited disorder affecting approximately 400,000 people globally, characterized by a deficiency of coagulation factors [1]. Hemophilia A, associated with factor VIII, occurs in about 1 in 5,000 male births, while hemophilia B, associated with factor IX, occurs in about 1 in 30,000 [2]. It is classified into three categories: severe (factor activity level: <1 IU/dl or <1%), moderate (1%-5%), and mild (5%-40%) [3].
In people with hemophilia (PWH), bleeding into the joints accounts for 80% to 90% of all bleeding episodes. This is especially true for those with severe hemophilia, where bleeding can occur spontaneously or after minimal stress or trauma [4, 5]. The knee joint is the most commonly affected area due to its large synovial membrane and the significant rotational forces involved [6].
Recurrent bleeding in the knee joint often leads to chronic hemophilic arthropathy (HA), characterized by pain, swelling, soft tissue contractures, and damage to cartilage and bone [7-9]. This cartilage destruction results from direct exposure to blood and inflammation in the synovium. Even brief exposure can cause lasting damage [10, 11]. The inflammation and synovial hypertrophy in HA resemble those in rheumatoid arthritis, while the degeneration of hyaline cartilage is similar to that seen in osteoarthritis. These overlapping processes can lead to progressive joint destruction [10, 12].
Most management recommendations for HA focus on pharmacological treatment and factor replacement therapy. The knees are the most commonly affected joints (45%) in patients who do not receive prophylactic treatment [6, 13]. Despite improved treatments to prevent recurrent joint bleeding, blood-induced HA remains a major source of morbidity in PWH, significantly affecting their quality of life [14]. In severe hemophilia, the first hemarthrosis typically occurs around age 2, and if untreated, it can lead to HA by age 20 [15].
Non-pharmacological management includes a variety of physical therapy interventions, such as isometric and isotonic exercises, strength and balance training, cycling, treadmill walking, hydrotherapy, manual therapy techniques, proprioceptive training, and physical activity [16-28]. These approaches have shown a potential to improve the quality of life in PWH by enhancing mobility and function. However, the limited number of studies and diverse outcomes prevent the confirmation of their efficacy. Therefore, further robust research is needed to evaluate rehabilitation and physiotherapy in managing chronic HA in PWH [29].
Muscle energy technique (MET) is a manual therapy that has been shown to increase the range of motion (ROM) and improve function in many joints. Post-isometric relaxation (PIR) is a type of MET that operates on the principle of autogenic inhibition to improve joint range and muscle strength. It involves applying increased tension to the muscle fibers by asking the patient to contract against the resistance of the therapist, activating the Golgi tendon organ, which leads to reflex inhibition and muscle relaxation, allowing for effective passive stretching [30, 31].
PIR is used in managing various musculoskeletal conditions based on the principles of restoring biomechanics and minimizing movement restrictions and pain [30-32]. A few studies demonstrate the effectiveness of MET on other musculoskeletal conditions, either as a stand-alone intervention or in combination with other therapeutic exercises, showing a positive therapeutic impact on joint mobility, muscle strength, and pain reduction, although they lack strong methodology [33]. To the best of our knowledge, no studies describe the application of PIR in HA, although it is extensively used for other musculoskeletal populations. Thus, this study aimed to prospectively assess the efficacy of PIR in addition to conventional physical therapy compared to conventional physical therapy alone in individuals with HA.
Materials and Methods
Study design and setting
This quasi-experimental study was conducted at a single center. Individuals with severe hemophilia A or B who had HA of the knee joint and were registered at the Hemophilia Treatment Care Center of the Hemophilia Society in South India were assessed for eligibility from June 2023 to May 2024.
Participants
To be eligible for inclusion, participants had to be diagnosed with HA of the unilateral knee joint of any duration and be between 18 and 40 years old. Participants who had experienced recent joint or muscle bleeds within one month in the lower limbs, had undergone knee surgeries, had FVIII or FIX inhibitors, had neurological conditions, or had cognitive impairments affecting functional levels, as well as those receiving regular physical therapy within three months before the treatment commenced, were excluded from the study.
Group allocation and blinding
The eligible participants were assigned to either the control group (n=25) or the experimental group (n=25) by an independent researcher using a convenience sampling method at the outpatient unit in physiotherapy. The control group received conventional physical therapy, while the experimental group received the PIR intervention in addition to conventional physical therapy. A baseline assessment of demographic data, health history, and outcomes—including knee ROM, muscle strength, joint health, and function—was conducted by an outcome assessor who was blinded to the group allocation and the treatment received by both groups (Figure 1).
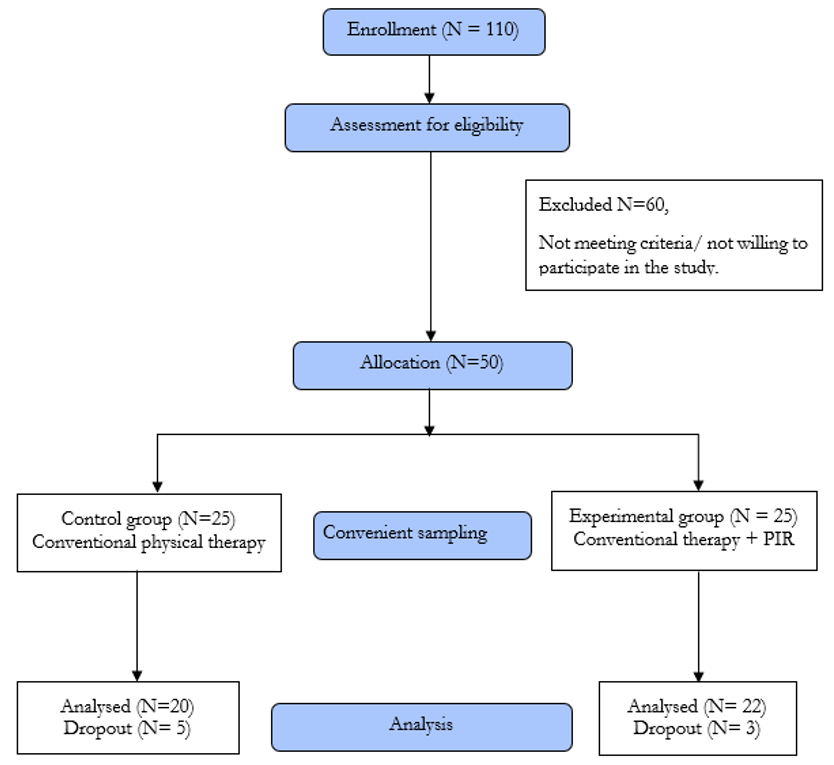
Figure 1. CONSORT flow diagram of participants
Outcome measures
The outcomes were measured before and after the physical therapy intervention for both the control and experimental groups. The knee ROM was assessed using a standard goniometer, which is widely used in clinical practice and has good intra-tester and inter-tester reliability [34]. The strength of the knee extensor and flexor muscles was quantified using the Lafayette Hand-Held Dynamometer (HHD), a valid assessment tool for objectively measuring muscle strength with high inter- and intra-rater reliability [35]. Joint health was measured using the Hemophilia Joint Health Score (HJHS), a validated scale for the physical examination of joint health in hemophilia, which demonstrates excellent inter-observer and test-retest reliability [36]. The total HJHS score was analyzed in this study. The Functional Independence Score in Hemophilia (FISH) was used to assess the participants’ independence in functional activities; this is a reliable tool developed and validated in a group of patients with significant HA [37].
Intervention
All 50 selected participants received conventional physical therapy for 30 minutes, three times a week for five consecutive weeks. In addition to this standard therapy, 25 out of the 50 participants were selected using convenience sampling. They received the PIR intervention for 15 minutes, three times a week for five consecutive weeks, alongside the conventional therapy. All interventions were conducted without prophylactic factor replacement therapy.
Conventional therapy
All 50 participants, including the control group (n=25) and the experimental group (n=25), received conventional therapy. Graded strengthening exercises were provided for the hip, knee, and ankle muscles. The exercises progressed from active free movements to resisted exercises, with resistance using weight cuffs being gradually increased each week, starting from 10 repetitions and gradually increasing to 20 repetitions. Active stretching of the hamstrings, hip flexors, and calf muscles was performed in a non-weight-bearing position, with the duration of the stretch increasing as tolerated by the patient, without inducing a joint bleed. Balance and proprioceptive training were conducted in weight-bearing positions, with or without the use of an assistive device as required. The exercise intensity was tailored according to the participants’ joint bleed history and pain levels [38].
PIR intervention
Of the 50 participants, 25 from the experimental group received PIR for the hamstrings and quadriceps muscles to improve knee extension and flexion ROM, respectively.
PIR for hamstring muscle (knee extension): In the supine position, the affected limb of the patient was lifted to assess the limitation in the knee extension range. At the point of restriction, the patient was instructed to press the leg downward into flexion using 10% of their maximal effort while the therapist resisted the movement (Figure 2). The patient was asked to hold this position for 7-8 seconds while contracting the hamstring muscle isometrically, and then to relax the muscle completely. The therapist then moved the limb up to a new barrier while the patient relaxed and repeated the same technique. This process was repeated 10 times.
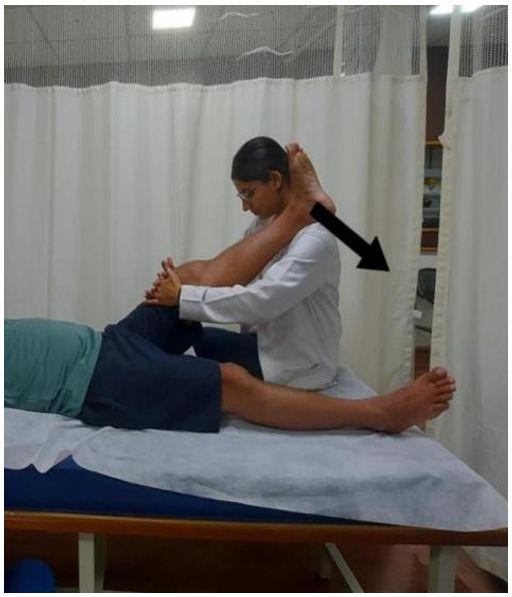
Figure 2. Post-isometric relaxation applied for hamstring muscles
PIR for quadriceps muscle (knee flexion): The patient was positioned in a prone lying position. The affected limb was passively bent at the knee joint by the therapist to assess the limitation in knee flexion. At the point of tissue restriction, the patient was instructed to press the limb into extension against the therapist’s resistance using 10% of their strength to contract isometrically (Figure 3). The patient was asked to hold this position for 7-8 seconds while contracting the quadriceps muscle isometrically and then to relax the muscle completely. While the patient relaxed, the therapist moved the limb into flexion to a new barrier and repeated the same technique. This process was repeated 10 times.
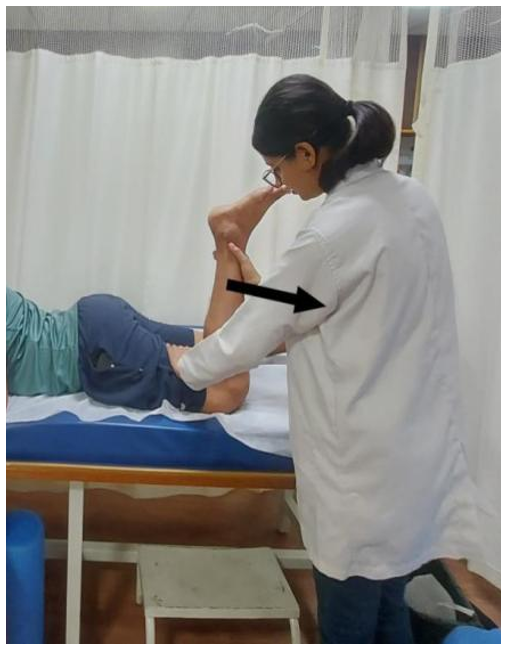
Figure 3. Post-isometric relaxation applied to the quadriceps muscle
Statistical analysis
SPSS version 29.0 was used to analyze the data. A one-sample t-test was employed to compare the average age of the groups and to analyze the pre- and post-test measurements of knee flexion, knee extension, quadriceps strength, and hamstring strength. Within each group, paired t-tests were utilized to assess changes over time. The Wilcoxon test was employed to compare the ordinal HJHS and FISH scores before and after the tests between the groups. Group results were contrasted using the Mann-Whitney U test. Pearson correlation analysis was used to compare the ROM, including flexion and extension, with dynamometry measurements for quadriceps and hamstring strength. The correlation between HJHS and FISH scores was assessed using Spearman’s correlation. Effect size was calculated using the mean difference, and the 95% confidence interval was determined for the outcomes. A p-value<0.05 was regarded as significant.
Findings
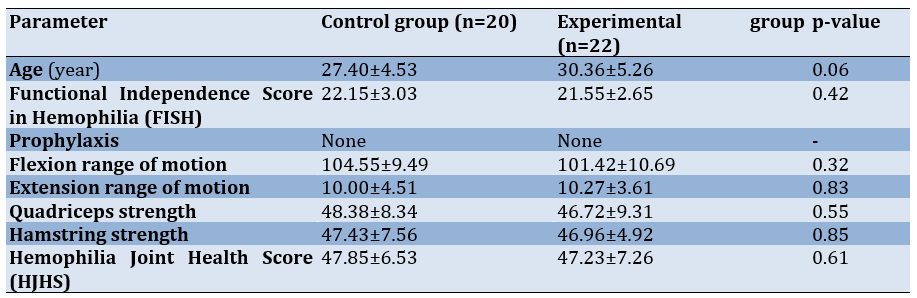
The data of 42 participants who completed five weeks of treatment sessions were included in the study, and their outcomes were analyzed. There were a total of eight dropouts (five from the control group and three from the experimental group) due to personal reasons (n=5) and non-compliance with the treatment protocol (n=3). The patients’ demographics, baseline characteristics, and outcomes were similar at baseline in both groups (p-value>0.05; Table 1). This ensured the homogeneity of the participants in the control and experimental groups. All participants were on episodic factor replacement therapy. In the control group, 19 cases (95%) had hemophilia A, while in the experimental group, all 22 cases (100%) had hemophilia A. In the control group, there was one case (0%) of hemophilia B, and there were no cases (0%) of hemophilia B in the experimental group.
Table 1. Mean baseline characteristics of participants with HA in the control and experimental groups
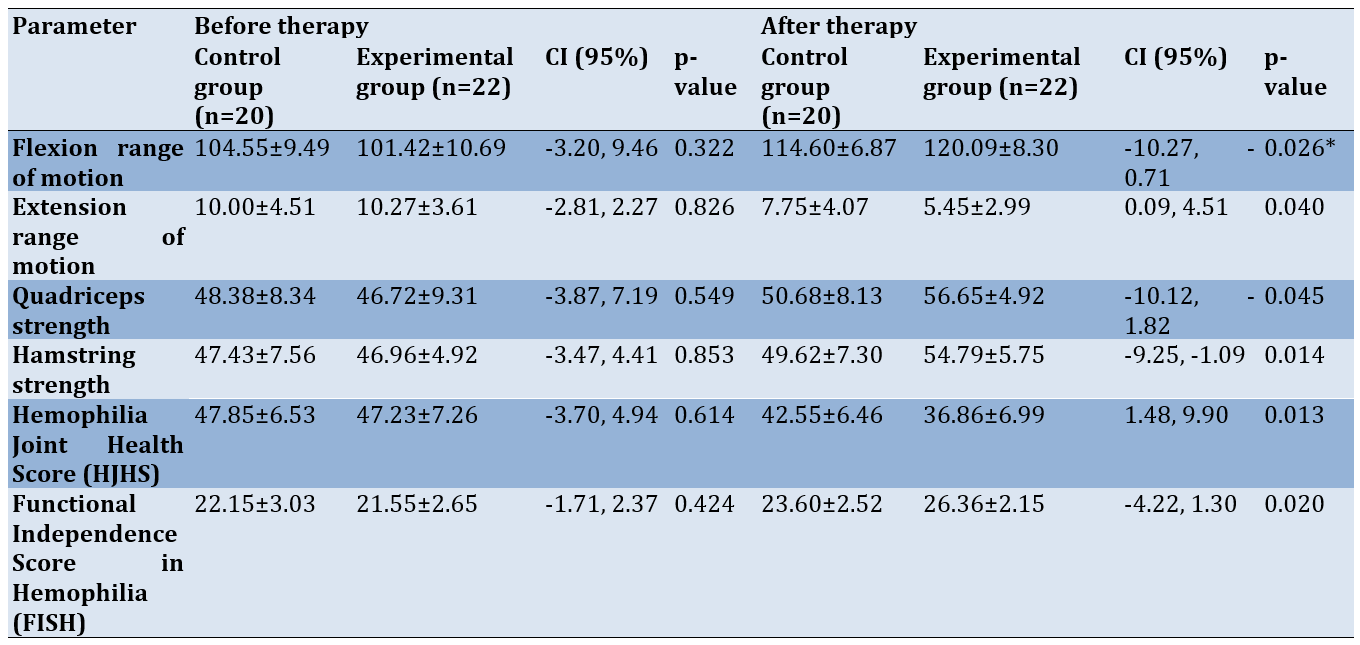
Both the control and experimental groups showed significant improvements in joint ROM, muscle strength, and overall joint health and function (p-value=0.001; Table 2).
Table 2. Comparison of mean outcomes before and after treatment in the control and experimental groups
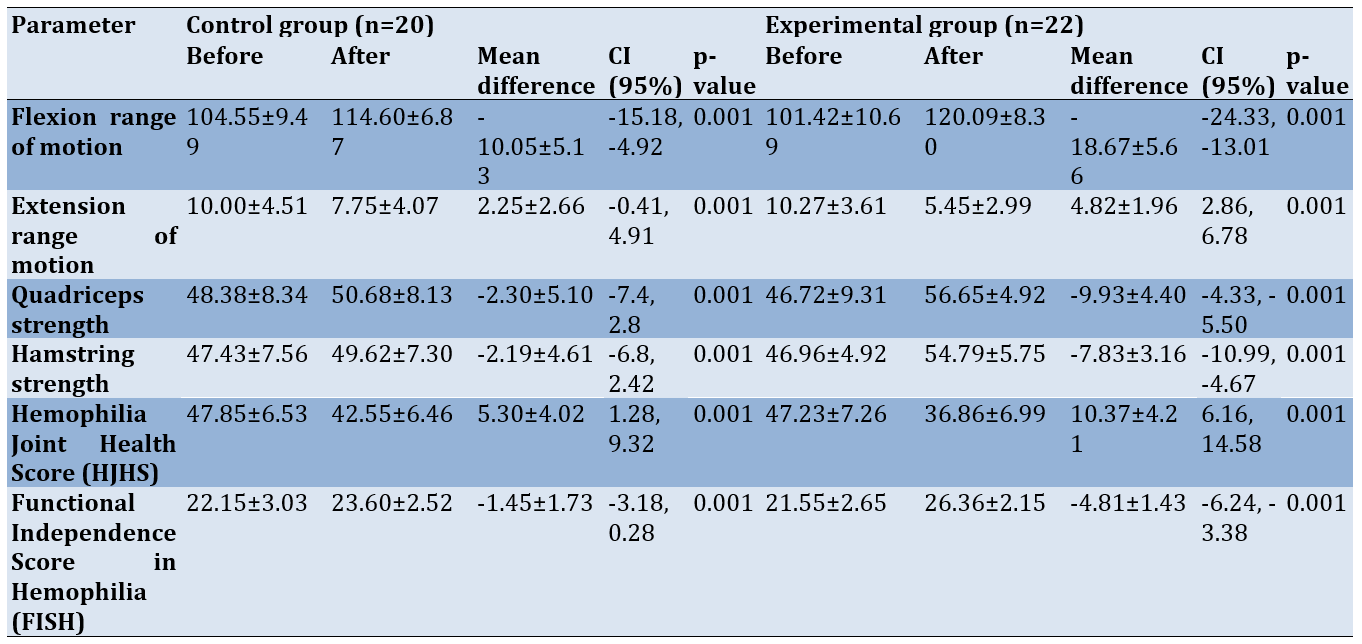
Baseline values for joint range, muscle strength, HJHS, and FISH for both groups were similar (p-value>0.05). Individuals who received PIR along with conventional therapy demonstrated significant improvements in knee flexion (mean difference [MD]=-18.67±5.66; 95% CI: -24.33, -13.01) and extension (MD=-4.82±1.96; 95% CI: -2.86, 6.78) range, muscle strength (extensors: MD=-9.93±4.40; 95% CI: -10.12, -1.82; flexors: MD = -7.83±3.16; 95% CI: -9.25, -1.09), joint health (MD=10.37±4.21; 95% CI: 6.16, 14.58), and joint function (MD = -4.81±1.43; 95% CI: -6.24, -3.38; Table 2) compared to those in the control group (p-value<0.05; Table 3).
Table 3. Comparison of mean outcomes before and after 5 weeks of intervention between the control and experimental groups
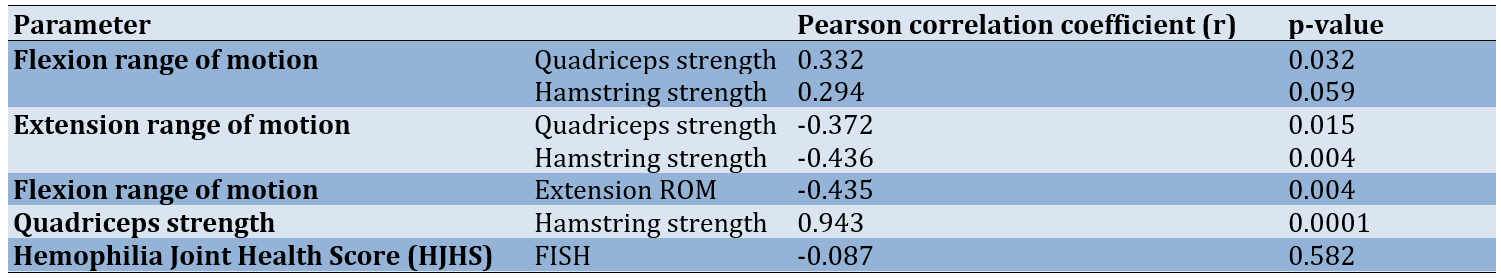
The correlation between knee ROM and knee muscle strength was found to be weakly positive to moderately negative. This suggests that as the range improves, muscle strength may increase. Flexion and extension ROM had a moderate negative correlation, indicating that as knee extension improves, flexion decreases. There was a strong positive correlation found between quadriceps and hamstring strength. This correlation may be attributed to the irradiation of muscles, where the strong contraction of one muscle group can activate nearby muscles, resulting in increased overall force production. A weak negative correlation was observed between joint health and function. This discrepancy arises because the HJHS score uses a negative scoring system, while the FISH utilizes a positive scale. However, this correlation was not statistically significant (Table 4).
Table 4. Correlation between outcome variables
Discussion
This quasi-experimental study aimed to prospectively compare the effects of PIR combined with conventional physical therapy versus conventional physical therapy alone, in persons with HA.
The mean ages of PWH and HA in the experimental and control groups at the start of treatment were 30.36±5.26 years and 27.40±4.53 years, respectively. This is consistent with other research showing that HA usually begins in early childhood and advances before age thirty [39]. Our study participants were all on episodic factor replacement therapy, which could have contributed to their early HA. Literature suggests that in the absence of prophylactic factor replacement therapy, individuals are likely to develop HA by the age of 20 [15]. The majority of participants had severe hemophilia A, which aligns with earlier findings that hemophilia A is more common than hemophilia B [2].
We observed a significant improvement in outcomes in both groups after five weeks of intervention. Similar to the experimental group that received both conventional therapy and PIR intervention, the control group, which received conventional therapy, also showed a significant improvement in their ROM, muscle strength, and overall joint health and function.
This finding is not surprising, as physical therapy has been shown to improve outcomes for PWH who have multiple joints affected by arthropathies [29]. We provided graded strengthening exercises using resistance, active stretching for tight muscles, balance training, and proprioceptive training. These activities were tailored according to each participant’s initial assessment to prevent the risk of inducing a joint bleed [38].
Resistance training has demonstrated significant improvements in various outcomes for PWH, according to existing literature [16, 23, 24, 29, 40]. A study comparing the effects of a progressive resistance training program with other exercise modalities on joint function in young PWH found that all groups receiving resistance training experience significant improvements in joint function [23]. Furthermore, some studies have documented that while improvements can occur with electrotherapy modalities, it is clear that exercise may enhance these effects [24]. According to a systematic review of the impact of exercise on musculoskeletal function and pain, it was concluded that supervised programs using resistance training or balance training may help improve muscle strength, ROM, and pain in PWH with HA [16, 17]. Additionally, it has been reported that physical therapy incorporating strength training, balance exercises, and proprioception significantly improves joint ROM, position sense, static and dynamic balance, and pain in individuals with chronic arthropathy, according to a comprehensive review [40].
Both the control and groups received traditional interventions, which may explain the improved outcomes observed in both groups. However, individuals who received PIR along with conventional therapy showed a significantly greater increase in their ROM, muscle strength, and overall joint health and function compared to those in the control group. When comparing the mean difference between the pre- and post-outcomes of the experimental group to the control group, the experimental group showed a higher mean difference than the control group.
The improvements in knee ROM observed in the experimental group that received PIR intervention may be due to the relaxation and lengthening of muscles that occur following an isometric contraction, a phenomenon known as autogenic inhibition. It is believed that after an isometric contraction, reflexive inhibition and relaxation of the muscle enable the clinician to perform a passive stretch because the muscle is in a refractory state [30, 31].
Our findings align with previous studies demonstrating that PIR is effective in improving outcomes in joint arthropathies [30-32]. Additionally, earlier studies have shown that MET, including PIR, whether as a stand-alone intervention or in combination with other therapeutic exercises, has a positive impact on joint mobility, muscle strength, and pain reduction [33].
There were no worsening of outcomes or adverse events reported by any individuals in both groups. HA is a significant morbidity associated with hemophilia, with a considerable impact on the quality of life for PWH [14], particularly affecting the knee in individuals who do not have access to prophylactic factor replacement therapy [6, 13]. The current study, along with previous research, suggests that PIR, when combined with conventional physical therapy interventions, may be effective for improving outcomes in the management of HA in PWH. However, the theoretical rationale for this clinical finding is not established based solely on this evidence.
Although this was a quasi-experimental study, several measures were implemented to reduce bias, including the use of an independent researcher to allocate individuals to the intervention groups, blinding the outcome assessor, and ensuring comparable baseline characteristics among participants. Despite these efforts, several limitations were identified in our study. Firstly, the lack of randomization may have introduced selection bias, as we could not deny treatment to PWH who wanted the PIR intervention. Secondly, the use of convenience sampling methods for participant assignment may reduce the external validity of the findings. Additionally, while only the outcome assessor was blinded, neither the therapists nor the participants were blinded, which could lead to potential performance bias. However, due to the nature of the intervention, blinding the study subjects and therapists was not feasible. Other limitations included the absence of a placebo or non-treatment group and the lack of long-term follow-up.
It is essential to conduct robust, well-designed randomized clinical studies to evaluate the effects of physical therapy, including the PIR intervention, in managing chronic HA in PWH. These studies should involve larger participant groups and include extended follow-up periods to assess long-term effectiveness. Currently, it is unclear whether the benefits of the physical therapy intervention persist over time. Despite these limitations, this is the first study to report the effect of the PIR intervention in individuals with severe hemophilia for the management of knee HA.
Conclusion
PIR is a useful and appropriate intervention for managing hemophilia-associated arthropathy in PWH.
Acknowledgments: We would like to thank Dr. Siju K. Paul for helping recruit the patients and the Research Department of Krupanidhi Group of Institutions for their statistical assistance.
Ethical Permissions: The study was reviewed and approved by the Institutional Ethics Committee of Krupanidhi College of Physiotherapy (EC-MPT/23/PHY/001), South India. Written consent was obtained by the principal investigator in the participants’ regional language.
Conflicts of Interests: The authors have no competing interests.
Authors' Contribution: Sharma S (First Author), Introduction Writer/Main Researcher/Discussion Writer (50%); Tilak Francis TG (Second Author), Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (50%)
Funding/Support: This study received no funding.
Hemophilia is an X-linked inherited disorder affecting approximately 400,000 people globally, characterized by a deficiency of coagulation factors [1]. Hemophilia A, associated with factor VIII, occurs in about 1 in 5,000 male births, while hemophilia B, associated with factor IX, occurs in about 1 in 30,000 [2]. It is classified into three categories: severe (factor activity level: <1 IU/dl or <1%), moderate (1%-5%), and mild (5%-40%) [3].
In people with hemophilia (PWH), bleeding into the joints accounts for 80% to 90% of all bleeding episodes. This is especially true for those with severe hemophilia, where bleeding can occur spontaneously or after minimal stress or trauma [4, 5]. The knee joint is the most commonly affected area due to its large synovial membrane and the significant rotational forces involved [6].
Recurrent bleeding in the knee joint often leads to chronic hemophilic arthropathy (HA), characterized by pain, swelling, soft tissue contractures, and damage to cartilage and bone [7-9]. This cartilage destruction results from direct exposure to blood and inflammation in the synovium. Even brief exposure can cause lasting damage [10, 11]. The inflammation and synovial hypertrophy in HA resemble those in rheumatoid arthritis, while the degeneration of hyaline cartilage is similar to that seen in osteoarthritis. These overlapping processes can lead to progressive joint destruction [10, 12].
Most management recommendations for HA focus on pharmacological treatment and factor replacement therapy. The knees are the most commonly affected joints (45%) in patients who do not receive prophylactic treatment [6, 13]. Despite improved treatments to prevent recurrent joint bleeding, blood-induced HA remains a major source of morbidity in PWH, significantly affecting their quality of life [14]. In severe hemophilia, the first hemarthrosis typically occurs around age 2, and if untreated, it can lead to HA by age 20 [15].
Non-pharmacological management includes a variety of physical therapy interventions, such as isometric and isotonic exercises, strength and balance training, cycling, treadmill walking, hydrotherapy, manual therapy techniques, proprioceptive training, and physical activity [16-28]. These approaches have shown a potential to improve the quality of life in PWH by enhancing mobility and function. However, the limited number of studies and diverse outcomes prevent the confirmation of their efficacy. Therefore, further robust research is needed to evaluate rehabilitation and physiotherapy in managing chronic HA in PWH [29].
Muscle energy technique (MET) is a manual therapy that has been shown to increase the range of motion (ROM) and improve function in many joints. Post-isometric relaxation (PIR) is a type of MET that operates on the principle of autogenic inhibition to improve joint range and muscle strength. It involves applying increased tension to the muscle fibers by asking the patient to contract against the resistance of the therapist, activating the Golgi tendon organ, which leads to reflex inhibition and muscle relaxation, allowing for effective passive stretching [30, 31].
PIR is used in managing various musculoskeletal conditions based on the principles of restoring biomechanics and minimizing movement restrictions and pain [30-32]. A few studies demonstrate the effectiveness of MET on other musculoskeletal conditions, either as a stand-alone intervention or in combination with other therapeutic exercises, showing a positive therapeutic impact on joint mobility, muscle strength, and pain reduction, although they lack strong methodology [33]. To the best of our knowledge, no studies describe the application of PIR in HA, although it is extensively used for other musculoskeletal populations. Thus, this study aimed to prospectively assess the efficacy of PIR in addition to conventional physical therapy compared to conventional physical therapy alone in individuals with HA.
Materials and Methods
Study design and setting
This quasi-experimental study was conducted at a single center. Individuals with severe hemophilia A or B who had HA of the knee joint and were registered at the Hemophilia Treatment Care Center of the Hemophilia Society in South India were assessed for eligibility from June 2023 to May 2024.
Participants
To be eligible for inclusion, participants had to be diagnosed with HA of the unilateral knee joint of any duration and be between 18 and 40 years old. Participants who had experienced recent joint or muscle bleeds within one month in the lower limbs, had undergone knee surgeries, had FVIII or FIX inhibitors, had neurological conditions, or had cognitive impairments affecting functional levels, as well as those receiving regular physical therapy within three months before the treatment commenced, were excluded from the study.
Group allocation and blinding
The eligible participants were assigned to either the control group (n=25) or the experimental group (n=25) by an independent researcher using a convenience sampling method at the outpatient unit in physiotherapy. The control group received conventional physical therapy, while the experimental group received the PIR intervention in addition to conventional physical therapy. A baseline assessment of demographic data, health history, and outcomes—including knee ROM, muscle strength, joint health, and function—was conducted by an outcome assessor who was blinded to the group allocation and the treatment received by both groups (Figure 1).

Figure 1. CONSORT flow diagram of participants
Outcome measures
The outcomes were measured before and after the physical therapy intervention for both the control and experimental groups. The knee ROM was assessed using a standard goniometer, which is widely used in clinical practice and has good intra-tester and inter-tester reliability [34]. The strength of the knee extensor and flexor muscles was quantified using the Lafayette Hand-Held Dynamometer (HHD), a valid assessment tool for objectively measuring muscle strength with high inter- and intra-rater reliability [35]. Joint health was measured using the Hemophilia Joint Health Score (HJHS), a validated scale for the physical examination of joint health in hemophilia, which demonstrates excellent inter-observer and test-retest reliability [36]. The total HJHS score was analyzed in this study. The Functional Independence Score in Hemophilia (FISH) was used to assess the participants’ independence in functional activities; this is a reliable tool developed and validated in a group of patients with significant HA [37].
Intervention
All 50 selected participants received conventional physical therapy for 30 minutes, three times a week for five consecutive weeks. In addition to this standard therapy, 25 out of the 50 participants were selected using convenience sampling. They received the PIR intervention for 15 minutes, three times a week for five consecutive weeks, alongside the conventional therapy. All interventions were conducted without prophylactic factor replacement therapy.
Conventional therapy
All 50 participants, including the control group (n=25) and the experimental group (n=25), received conventional therapy. Graded strengthening exercises were provided for the hip, knee, and ankle muscles. The exercises progressed from active free movements to resisted exercises, with resistance using weight cuffs being gradually increased each week, starting from 10 repetitions and gradually increasing to 20 repetitions. Active stretching of the hamstrings, hip flexors, and calf muscles was performed in a non-weight-bearing position, with the duration of the stretch increasing as tolerated by the patient, without inducing a joint bleed. Balance and proprioceptive training were conducted in weight-bearing positions, with or without the use of an assistive device as required. The exercise intensity was tailored according to the participants’ joint bleed history and pain levels [38].
PIR intervention
Of the 50 participants, 25 from the experimental group received PIR for the hamstrings and quadriceps muscles to improve knee extension and flexion ROM, respectively.
PIR for hamstring muscle (knee extension): In the supine position, the affected limb of the patient was lifted to assess the limitation in the knee extension range. At the point of restriction, the patient was instructed to press the leg downward into flexion using 10% of their maximal effort while the therapist resisted the movement (Figure 2). The patient was asked to hold this position for 7-8 seconds while contracting the hamstring muscle isometrically, and then to relax the muscle completely. The therapist then moved the limb up to a new barrier while the patient relaxed and repeated the same technique. This process was repeated 10 times.

Figure 2. Post-isometric relaxation applied for hamstring muscles
PIR for quadriceps muscle (knee flexion): The patient was positioned in a prone lying position. The affected limb was passively bent at the knee joint by the therapist to assess the limitation in knee flexion. At the point of tissue restriction, the patient was instructed to press the limb into extension against the therapist’s resistance using 10% of their strength to contract isometrically (Figure 3). The patient was asked to hold this position for 7-8 seconds while contracting the quadriceps muscle isometrically and then to relax the muscle completely. While the patient relaxed, the therapist moved the limb into flexion to a new barrier and repeated the same technique. This process was repeated 10 times.

Figure 3. Post-isometric relaxation applied to the quadriceps muscle
Statistical analysis
SPSS version 29.0 was used to analyze the data. A one-sample t-test was employed to compare the average age of the groups and to analyze the pre- and post-test measurements of knee flexion, knee extension, quadriceps strength, and hamstring strength. Within each group, paired t-tests were utilized to assess changes over time. The Wilcoxon test was employed to compare the ordinal HJHS and FISH scores before and after the tests between the groups. Group results were contrasted using the Mann-Whitney U test. Pearson correlation analysis was used to compare the ROM, including flexion and extension, with dynamometry measurements for quadriceps and hamstring strength. The correlation between HJHS and FISH scores was assessed using Spearman’s correlation. Effect size was calculated using the mean difference, and the 95% confidence interval was determined for the outcomes. A p-value<0.05 was regarded as significant.
Findings
The data of 42 participants who completed five weeks of treatment sessions were included in the study, and their outcomes were analyzed. There were a total of eight dropouts (five from the control group and three from the experimental group) due to personal reasons (n=5) and non-compliance with the treatment protocol (n=3). The patients’ demographics, baseline characteristics, and outcomes were similar at baseline in both groups (p-value>0.05; Table 1). This ensured the homogeneity of the participants in the control and experimental groups. All participants were on episodic factor replacement therapy. In the control group, 19 cases (95%) had hemophilia A, while in the experimental group, all 22 cases (100%) had hemophilia A. In the control group, there was one case (0%) of hemophilia B, and there were no cases (0%) of hemophilia B in the experimental group.
Table 1. Mean baseline characteristics of participants with HA in the control and experimental groups

Both the control and experimental groups showed significant improvements in joint ROM, muscle strength, and overall joint health and function (p-value=0.001; Table 2).
Table 2. Comparison of mean outcomes before and after treatment in the control and experimental groups

Baseline values for joint range, muscle strength, HJHS, and FISH for both groups were similar (p-value>0.05). Individuals who received PIR along with conventional therapy demonstrated significant improvements in knee flexion (mean difference [MD]=-18.67±5.66; 95% CI: -24.33, -13.01) and extension (MD=-4.82±1.96; 95% CI: -2.86, 6.78) range, muscle strength (extensors: MD=-9.93±4.40; 95% CI: -10.12, -1.82; flexors: MD = -7.83±3.16; 95% CI: -9.25, -1.09), joint health (MD=10.37±4.21; 95% CI: 6.16, 14.58), and joint function (MD = -4.81±1.43; 95% CI: -6.24, -3.38; Table 2) compared to those in the control group (p-value<0.05; Table 3).
Table 3. Comparison of mean outcomes before and after 5 weeks of intervention between the control and experimental groups

The correlation between knee ROM and knee muscle strength was found to be weakly positive to moderately negative. This suggests that as the range improves, muscle strength may increase. Flexion and extension ROM had a moderate negative correlation, indicating that as knee extension improves, flexion decreases. There was a strong positive correlation found between quadriceps and hamstring strength. This correlation may be attributed to the irradiation of muscles, where the strong contraction of one muscle group can activate nearby muscles, resulting in increased overall force production. A weak negative correlation was observed between joint health and function. This discrepancy arises because the HJHS score uses a negative scoring system, while the FISH utilizes a positive scale. However, this correlation was not statistically significant (Table 4).
Table 4. Correlation between outcome variables

Discussion
This quasi-experimental study aimed to prospectively compare the effects of PIR combined with conventional physical therapy versus conventional physical therapy alone, in persons with HA.
The mean ages of PWH and HA in the experimental and control groups at the start of treatment were 30.36±5.26 years and 27.40±4.53 years, respectively. This is consistent with other research showing that HA usually begins in early childhood and advances before age thirty [39]. Our study participants were all on episodic factor replacement therapy, which could have contributed to their early HA. Literature suggests that in the absence of prophylactic factor replacement therapy, individuals are likely to develop HA by the age of 20 [15]. The majority of participants had severe hemophilia A, which aligns with earlier findings that hemophilia A is more common than hemophilia B [2].
We observed a significant improvement in outcomes in both groups after five weeks of intervention. Similar to the experimental group that received both conventional therapy and PIR intervention, the control group, which received conventional therapy, also showed a significant improvement in their ROM, muscle strength, and overall joint health and function.
This finding is not surprising, as physical therapy has been shown to improve outcomes for PWH who have multiple joints affected by arthropathies [29]. We provided graded strengthening exercises using resistance, active stretching for tight muscles, balance training, and proprioceptive training. These activities were tailored according to each participant’s initial assessment to prevent the risk of inducing a joint bleed [38].
Resistance training has demonstrated significant improvements in various outcomes for PWH, according to existing literature [16, 23, 24, 29, 40]. A study comparing the effects of a progressive resistance training program with other exercise modalities on joint function in young PWH found that all groups receiving resistance training experience significant improvements in joint function [23]. Furthermore, some studies have documented that while improvements can occur with electrotherapy modalities, it is clear that exercise may enhance these effects [24]. According to a systematic review of the impact of exercise on musculoskeletal function and pain, it was concluded that supervised programs using resistance training or balance training may help improve muscle strength, ROM, and pain in PWH with HA [16, 17]. Additionally, it has been reported that physical therapy incorporating strength training, balance exercises, and proprioception significantly improves joint ROM, position sense, static and dynamic balance, and pain in individuals with chronic arthropathy, according to a comprehensive review [40].
Both the control and groups received traditional interventions, which may explain the improved outcomes observed in both groups. However, individuals who received PIR along with conventional therapy showed a significantly greater increase in their ROM, muscle strength, and overall joint health and function compared to those in the control group. When comparing the mean difference between the pre- and post-outcomes of the experimental group to the control group, the experimental group showed a higher mean difference than the control group.
The improvements in knee ROM observed in the experimental group that received PIR intervention may be due to the relaxation and lengthening of muscles that occur following an isometric contraction, a phenomenon known as autogenic inhibition. It is believed that after an isometric contraction, reflexive inhibition and relaxation of the muscle enable the clinician to perform a passive stretch because the muscle is in a refractory state [30, 31].
Our findings align with previous studies demonstrating that PIR is effective in improving outcomes in joint arthropathies [30-32]. Additionally, earlier studies have shown that MET, including PIR, whether as a stand-alone intervention or in combination with other therapeutic exercises, has a positive impact on joint mobility, muscle strength, and pain reduction [33].
There were no worsening of outcomes or adverse events reported by any individuals in both groups. HA is a significant morbidity associated with hemophilia, with a considerable impact on the quality of life for PWH [14], particularly affecting the knee in individuals who do not have access to prophylactic factor replacement therapy [6, 13]. The current study, along with previous research, suggests that PIR, when combined with conventional physical therapy interventions, may be effective for improving outcomes in the management of HA in PWH. However, the theoretical rationale for this clinical finding is not established based solely on this evidence.
Although this was a quasi-experimental study, several measures were implemented to reduce bias, including the use of an independent researcher to allocate individuals to the intervention groups, blinding the outcome assessor, and ensuring comparable baseline characteristics among participants. Despite these efforts, several limitations were identified in our study. Firstly, the lack of randomization may have introduced selection bias, as we could not deny treatment to PWH who wanted the PIR intervention. Secondly, the use of convenience sampling methods for participant assignment may reduce the external validity of the findings. Additionally, while only the outcome assessor was blinded, neither the therapists nor the participants were blinded, which could lead to potential performance bias. However, due to the nature of the intervention, blinding the study subjects and therapists was not feasible. Other limitations included the absence of a placebo or non-treatment group and the lack of long-term follow-up.
It is essential to conduct robust, well-designed randomized clinical studies to evaluate the effects of physical therapy, including the PIR intervention, in managing chronic HA in PWH. These studies should involve larger participant groups and include extended follow-up periods to assess long-term effectiveness. Currently, it is unclear whether the benefits of the physical therapy intervention persist over time. Despite these limitations, this is the first study to report the effect of the PIR intervention in individuals with severe hemophilia for the management of knee HA.
Conclusion
PIR is a useful and appropriate intervention for managing hemophilia-associated arthropathy in PWH.
Acknowledgments: We would like to thank Dr. Siju K. Paul for helping recruit the patients and the Research Department of Krupanidhi Group of Institutions for their statistical assistance.
Ethical Permissions: The study was reviewed and approved by the Institutional Ethics Committee of Krupanidhi College of Physiotherapy (EC-MPT/23/PHY/001), South India. Written consent was obtained by the principal investigator in the participants’ regional language.
Conflicts of Interests: The authors have no competing interests.
Authors' Contribution: Sharma S (First Author), Introduction Writer/Main Researcher/Discussion Writer (50%); Tilak Francis TG (Second Author), Methodologist/Assistant Researcher/Discussion Writer/Statistical Analyst (50%)
Funding/Support: This study received no funding.
Article Type: Original Research |
Subject:
Healthy Life Style
Received: 2025/01/4 | Accepted: 2025/02/19 | Published: 2025/04/26
Received: 2025/01/4 | Accepted: 2025/02/19 | Published: 2025/04/26
References
1. Alcalay M, Deplas A. Rheumatogical management of patients with hemophilia. Part II: Muscle haematomas and pseudotumors. Joint Bone Spine. 2002;69(6):556-9. [Link] [DOI:10.1016/S1297-319X(02)00451-7]
2. Rodriguez-Merchan EC, Goddard NJ. Muscular bleeding, soft tissue haematomas and pseudotumours. In: Musculoskeletal aspects of hemophilia. Hoboken: John Wiley & Sons; 2000. [Link] [DOI:10.1002/9780470693872.ch13]
3. Llinas A. Hemophilic arthropathy. Hemophilia. 2010;16(Suppl 5):121. [Link] [DOI:10.1111/j.1365-2516.2010.02309_1.x]
4. Rodriguez-Merchan EC. Musculoskeletal complications of hemophilia. HSS J. 2010;6(1):37-42. [Link] [DOI:10.1007/s11420-009-9140-9]
5. Seuser A, Djambas Khayat C, Negrier C, Sabbour A, Heijnen L. Evaluation of early musculoskeletal disease in patients with hemophilia: Results from an expert consensus. Blood Coagul Fibrinolysis. 2018;29(6):509-20. [Link] [DOI:10.1097/MBC.0000000000000767]
6. Rodriguez-Merchan EC. Pathogenesis, early diagnosis, and prophylaxis for chronic hemophilic synovitis. Clin Orthop Relat Res. 1997;343:6-11. [Link] [DOI:10.1097/00003086-199710000-00003]
7. Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977;59(3):287-305. [Link] [DOI:10.2106/00004623-197759030-00001]
8. Balci HI, Kocaoglu M, Eralp L, Bilen FE. Knee flexion contracture in hemophilia: Treatment with circular external fixator. Hemophilia. 2014;20(6):879-83. [Link] [DOI:10.1111/hae.12478]
9. Kiely PD, McMahon C, Smith OP, Moore DP. The treatment of flexion contracture of the knee using the Ilizarov technique in a child with hemophilia B. Hemophilia. 2003;9(3):336-9. [Link] [DOI:10.1046/j.1365-2516.2003.00753.x]
10. Lafeber FPGJ, Miossec P, Valentino LA. Physiopathology of hemophilic arthropathy. Hemophilia. 2008;14(Suppl 4):3-9. [Link] [DOI:10.1111/j.1365-2516.2008.01732.x]
11. Jansen NW, Roosendaal G, Bijlsma JW, Degroot J, Lafeber FP. Exposure of human cartilage tissue to low concentrations of blood for a short period of time leads to prolonged cartilage damage: An in vitro study. Arthritis Rheum. 2007;56(1):199-207. [Link] [DOI:10.1002/art.22304]
12. Roosendaal G, Van Rinsum AC, Vianen ME, Van Den Berg HM, Lafeber FP, Bijlsma JW. Hemophilic arthropathy resembles degenerative rather than inflammatory joint disease. Histopathology. 1999;34(2):144-53. [Link] [DOI:10.1046/j.1365-2559.1999.00608.x]
13. Srivastava A, Brewer AK, Mauser-Bunschoten EP, Key NS, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Hemophilia. 2013;19(1):e1-47. [Link] [DOI:10.1111/j.1365-2516.2012.02909.x]
14. Wyseure T, Mosnier LO, Von Drygalski A. Advances and challenges in hemophilic arthropathy. Semin Hematol. 2016;53(1):10-9. [Link] [DOI:10.1053/j.seminhematol.2015.10.005]
15. Blanchette P, Rivard G, Israels S, Robinson S, Ali K, Walker I, et al. A survey of factor prophylaxis in the Canadian hemophilia A population. Hemophilia. 2004;10(6):679-83. [Link] [DOI:10.1111/j.1365-2516.2004.01045.x]
16. Schäfer GS, Valderramas S, Gomes AR, Budib MB, Wolff ÁL, Ramos AA. Physical exercise, pain and musculoskeletal function in patients with hemophilia: A systematic review. Hemophilia. 2016;22(3):e119-29. [Link] [DOI:10.1111/hae.12909]
17. Strike K, Mulder K, Michael R. Exercise for hemophilia. Cochrane Database Syst Rev. 2016;12(12):CD011180. [Link] [DOI:10.1002/14651858.CD011180.pub2]
18. Cuesta-Barriuso R, Gómez-Conesa A, López-Pina JA. Manual therapy in the treatment of ankle hemophilic arthropathy. A randomized pilot study. Physiother Theory Pract. 2014;30(8):534-9. [Link] [DOI:10.3109/09593985.2014.902148]
19. Cuesta-Barriuso R, Gómez-Conesa A, López-Pina JA. Effectiveness of two modalities of physiotherapy in the treatment of Hemophilic arthropathy of the ankle: A randomized pilot study. Hemophilia. 2014;20(1):e71-8. [Link] [DOI:10.1111/hae.12320]
20. Scaddan E, Rowell J, O'Leary S. A preliminary case series evaluating the safety and immediate to short-term clinical benefits of joint mobilization in hemophilic arthritis of the lower limb. J Man Manip Ther. 2017;25(4):208-14. [Link] [DOI:10.1080/10669817.2016.1256117]
21. Donoso-Úbeda E, Meroño-Gallut J, López-Pina JA, Cuesta-Barriuso R. Safety and effectiveness of fascial therapy in adult patients with hemophilic arthropathy. A pilot study. Physiother Theory Pract. 2018;34(10):757-64. [Link] [DOI:10.1080/09593985.2018.1425513]
22. Cuesta-Barriuso R, Torres-Ortuño A, Nieto-Munuera J, López-Pina JA. Effectiveness of an educational physiotherapy and therapeutic exercise program in adult patients with hemophilia: A randomized controlled trial. Arch Phys Med Rehabil. 2017;98(5):841-8. [Link] [DOI:10.1016/j.apmr.2016.10.014]
23. Parhampour B, Torkaman G, Hoorfar H, Hedayati M, Ravanbod R. Effects of short-term resistance training and pulsed electromagnetic fields on bone metabolism and joint function in severe hemophilia A patients with osteoporosis: A randomized controlled trial. Clin Rehabil. 2014;28(5):440-50. [Link] [DOI:10.1177/0269215513505299]
24. El-Shamy SM, Abdelaal AAM. Efficacy of pulsed high-intensity laser therapy on pain, functional capacity, and gait in children with Hemophilic arthropathy. Disabil Rehabil. 2018;40(4):462-8. [Link] [DOI:10.1080/09638288.2016.1261416]
25. Buzzard B, Heim M. A study to evaluate the effectiveness of Air Stirrup splints as a means of reducing the frequency of ankle haemarthrosis in children with hemophilia A and B. Hemophilia. 1995;1(2):131-6. [Link] [DOI:10.1111/j.1365-2516.1995.tb00054.x]
26. Negrier C, Seuser A, Forsyth A, Lobet S, Llinas A, Rosas M, et al. The benefits of exercise for patients with hemophilia and recommendations for safe and effective physical activity. Hemophilia. 2013;19(4):487-98. [Link] [DOI:10.1111/hae.12118]
27. Runkel B, Von Mackensen S, Hilberg T. RCT-subjective physical performance and quality of life after a 6-month programmed sports therapy (PST) in patients with haemophilia. Haemophilia. 2017;23(1):144-51. [Link] [DOI:10.1111/hae.13079]
28. Runkel B, Czepa D, Hilberg T. RCT of a 6-month programmed sports therapy (PST) in patients with hemophilia-Improvement of physical fitness. Hemophilia. 2016;22:765-71. [Link] [DOI:10.1111/hae.12957]
29. Stephensen D, Bladen M, McLaughlin P. Recent advances in musculoskeletal physiotherapy for hemophilia. Ther Adv Hematol. 2018;9(8):227-37. [Link] [DOI:10.1177/2040620718784834]
30. Lewit K, Simons DG. Myofascial pain: Relief by post-isometric relaxation. Arch Phys Med Rehabil. 1984;65(8):452-6. [Link]
31. Khan ZK, Ahmed SI, Baig AAM, Farooqui WA. Effect of post-isometric relaxation versus myofascial release therapy on pain, functional disability, rom and QOL in the management of non-specific neck pain: A randomized controlled trial. BMC Musculoskelet Disord. 2022;23(1):567. [Link] [DOI:10.1186/s12891-022-05516-1]
32. Noto-Bell L, Vogel BN, Senn DE. Effects of post-isometric relaxation on ankle plantarflexion and timed flutter kick in pediatric competitive swimmers. J Am Osteopath Assoc. 2019;119(9):569-77. [Link] [DOI:10.7556/jaoa.2019.100]
33. Thomas E, Cavallaro AR, Mani D, Bianco A, Palma A. The efficacy of muscle energy techniques in symptomatic and asymptomatic subjects: A systematic review. Chiropr Man Therap. 2019;27(1):35. [Link] [DOI:10.1186/s12998-019-0258-7]
34. Gajdosik RL, Bohannon RW. Clinical measurement of range of motion: Review of goniometry emphasis reliability and validity. Phys Ther. 1987;67(12):1867-72. [Link] [DOI:10.1093/ptj/67.12.1867]
35. Chen B, Liu L, Bin Chen L, Cao X, Han P, Wang C, et al. Concurrent validity and reliability of a handheld dynamometer in measuring isometric shoulder rotational strength. J Sport Rehabil. 2021;30(6):965-8. [Link] [DOI:10.1123/jsr.2020-0021]
36. St-Louis J, Abad A, Funk S, Tilak M, Classey S, Zourikian N, et al. The hemophilia joint health score version 2.1 validation in adult patients study: A multicenter international study. Res Pract Thromb Haemost. 2022;6(2):e12690. [Link] [DOI:10.1002/rth2.12690]
37. Poonnoose PM, Thomas R, Keshava SN, Cherian RS, Padankatti S, Pazani D, et al. Psychometric analysis of the functional independence score in hemophilia (FISH). Hemophilia. 2007;13(5):620-6. [Link] [DOI:10.1111/j.1365-2516.2007.01508.x]
38. Tilak M, John JA, Paul A, Srivastava A, Singh D, Rajendran A, et al. Non-surgical correction of knee flexion deformity in persons with hemophilia: A staged multidisciplinary approach. Hemophilia. 2024;30(2):523-30. [Link] [DOI:10.1111/hae.14940]
39. Liu S, Zhou RF, Jin ZB, Wu M, Zhang PY. Age-related severity and distribution of hemophilic arthropathy of the knee, ankle and elbow among Chinese patients with hemophilia. Hemophilia. 2020;26(1):129-35. [Link] [DOI:10.1111/hae.13858]
40. Cuesta-Barriuso R, Gómez-Conesa A, López-Pina JA. Physiotherapy treatment in patients with hemophilia and chronic ankle arthropathy: A systematic review. Rehabil Res Pract. 2013;2013:305249. [Link] [DOI:10.1155/2013/305249]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






