Volume 12, Issue 3 (2024)
Health Educ Health Promot 2024, 12(3): 471-476 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Samami M, Salari A, Sharami S, Jafari Sadatmahalle Z, Najar-Karimi F. Frequency of Pregnancy Tumors and Associated Factors in the Oral Cavity of Pregnant Women in Northern Iran. Health Educ Health Promot 2024; 12 (3) :471-476
URL: http://hehp.modares.ac.ir/article-5-75766-en.html
URL: http://hehp.modares.ac.ir/article-5-75766-en.html
1- Department of Oral and Maxillofacial Medicine, School of Dentistry, Guilan University of Medical Sciences, Rasht, Iran
2- “Dental Sciences Research Center” and “Department of Periodontics, School of Dentistry”, Guilan University of Medical Sciences, Rasht, Iran
3- “Reproductive Health Research Center” and “Department of Obstetrics and Gynecology, Al-Zahra Hospital, School of Medicine”, Guilan University of Medical Sciences, Rasht, Iran
4- Department of Oral and Maxillofacial Medicine, School of Dentistry, Alborz University of Medical Sciences, Karaj, Iran
2- “Dental Sciences Research Center” and “Department of Periodontics, School of Dentistry”, Guilan University of Medical Sciences, Rasht, Iran
3- “Reproductive Health Research Center” and “Department of Obstetrics and Gynecology, Al-Zahra Hospital, School of Medicine”, Guilan University of Medical Sciences, Rasht, Iran
4- Department of Oral and Maxillofacial Medicine, School of Dentistry, Alborz University of Medical Sciences, Karaj, Iran
Full-Text [PDF 615 kb]
(2157 Downloads)
| Abstract (HTML) (972 Views)
Full-Text: (191 Views)
Introduction
The rising incidence of oral and dental diseases is a major concern for pregnant women, as it adversely affects their health and quality of life and poses risks for the fetus, including low birth weight [1]. Pyogenic granuloma (PG) is a benign vascular lesion that often grows rapidly and tends to bleed, predominantly found in the oral cavity, especially on the gums [2]. A specific type of PG, known as pregnancy tumor (PT) or granuloma gravidarum, commonly develops in pregnant women between the third and ninth months of pregnancy. Its size often increases toward the end of pregnancy due to hormonal changes, particularly elevated estrogen levels [3, 4]. Clinically, PT appears as an exophytic lesion with a smooth or lobulated surface, often ulcerated and reddish, usually pedunculated, and ranges in size from a few millimeters to up to 2 centimeters. It bleeds easily, even with minor irritation, such as brushing teeth. Although it can occur anywhere in the oral cavity, it is more prevalent on the upper gum, leading to secondary complications due to challenges in maintaining oral hygiene because of excessive bleeding. These complications include increased pocket depth, difficulty in removing microbial plaque, higher rates of dental caries, periodontal diseases, and an elevated risk of oral infections [2, 3].
Since this lesion is reactive and exacerbated by hormonal changes during pregnancy, such as increased estrogen activity on vascular tissues, along with chronic local irritants like tartar, dental plaque, broken and defective restorations, sharp teeth, and poor oral hygiene, it can be prevented by maintaining good oral hygiene and undergoing prophylactic treatments during pregnancy [2, 3].
If the PT is large, it can interfere with eating or brushing the teeth of pregnant women and cause severe discomfort due to bleeding and pain, which can lead to insufficient nutrition for the mother and inadequate development of the fetus, necessitating surgical removal. However, if patients are aware of how to prevent or control this lesion and if the size of the PT is small, it may heal spontaneously due to the sudden drop in estrogen levels after delivery [5, 6]. Therefore, it is essential to know the prevalence of this lesion in order to plan for its prevention and control of the aggravating factors.
Given the significant cultural differences in oral hygiene practices across various communities and the need to understand the prevalence of PT lesions for planning appropriate preventive measures in oral health education programs for pregnant women, it is essential to determine the prevalence of these lesions in the target population. Although various studies have examined the prevalence of these lesions [3, 4, 7], there is no consensus on the reported percentages across different communities. Due to the scarcity and outdated nature of studies and the lack of recent statistics in Gilan province, this study aimed to investigate the frequency of PTs and associated factors among pregnant women attending Al-Zahra Hospital in Rasht.
Instrument and Methods
Design
This cross-sectional analytical study included pregnant women who visited Al-Zahra Hospital in Rasht during the first, second, or third trimesters from 2022 to 2023, without any limitations on age, occupation, or other factors. The participants’ pregnancies were confirmed through urine HCG tests, supplementary blood tests, and ultrasounds.
Inclusion and exclusion criteria
Individuals with hormonal abnormalities, such as hyperthyroidism, specific infectious or systemic diseases, like HIV or diabetes, a history of malignancies, or concurrent tooth mobility or suspicious symptoms, like paresthesia alongside the exophytic lesion were excluded from the study. Any red to pink, pedunculated, or non-pedunculated lesions with soft to firm consistency that developed on the gums at any time during pregnancy were considered suspicious for PTs and included in the study [8]. The diagnosis of PTs was based on clinical appearance and histopathological examination.
Sampling
Sampling was conducted using simple random sampling. Samples were selected based on the last three digits of the medical records of patients visiting Al-Zahra Hospital, utilizing a random number table. The required sample size, based on the main objective and the estimated prevalence from the study by Patil [9], considering α=0.05 and d=0.02, was at least 707.
Data collection
Each patient completed a form with basic individual, demographic, and clinical information, including age, education level (below diploma/diploma/university degree), place of residence (urban/rural), medical and dental history, pregnancy duration (first, second, or third trimester), number of pregnancies, and the interval between pregnancies (in years).
Clinical examinations were performed using a “Williams” graduated probe and a disposable dental mirror (Brilliant model, Teksan Co., Iran) under adequate lighting. The buccal and lingual gums of the upper and lower jaws were examined by a final-year dental student under the supervision of an oral and maxillofacial specialist. Observations were recorded on a pre-prepared checklist by the researcher, which included the presence and location of the lesion, gingival inflammation (present/absent), the average number of quadrants with gingival inflammation, the bleeding on probing (BOP) index (positive/negative), plaque index (PI; ranging from 0 to 3 and average PI), frequency of daily use of a toothbrush, floss, and mouthwash, and the presence of inappropriate dental crowns/restorations in the lesion area.
After a thorough clinical examination of the intraoral and extraoral mucosa, any suspicious PTs identified were biopsied for further examination by an oral and maxillofacial specialist. The biopsied samples, after histopathological review and confirmation of PG by an oral pathologist, were included in the study. The BOP index was used to assess active gingivitis, evaluated based on clinical bleeding from the gingival margin using a Williams probe with appropriate pressure (0.75N, approximately 20-25g) and observing for bleeding within 10 seconds, ensuring that probing depth did not exceed 3mm at any point in the mouth. The Silness & Loe PI was also utilized [10-12].
Data analysis
Descriptive statistics for qualitative data were reported as frequencies and percentages, while quantitative data were presented as means and standard deviations. The Kolmogorov-Smirnov test was used to check the normality of the distribution of the groups. Depending on the type of parameter, the independent t-test and Chi-square test were used to compare groups if the assumptions were met; otherwise, Mann-Whitney U and Fisher’s exact tests were employed. Logistic regression analysis was used to simultaneously examine independent parameters and adjust for potential confounding factors. Data were analyzed using SPSS 28, at a significance level of p-value≤0.05.
Findings
Among the 707 pregnant women examined, with a mean age of 29.30±4.87 years, six cases (0.8%) had PT lesions (mean age=30.00±4.98 years), while 701 women (98.2%) were without lesions (mean age=28.60±4.77 years).
The average age of the group with PT was 30.00±4.98 years, and the average age of the group without PT was 28.60±4.77 years, with no significant difference between the two groups (p-value=0.473). Additionally, the average interval between pregnancies for the group with PT was 2.67±4.13 years, while for the group without PT, it was 30.00±4.98 years, and there was no significant difference between the two groups (p-value=0.376).
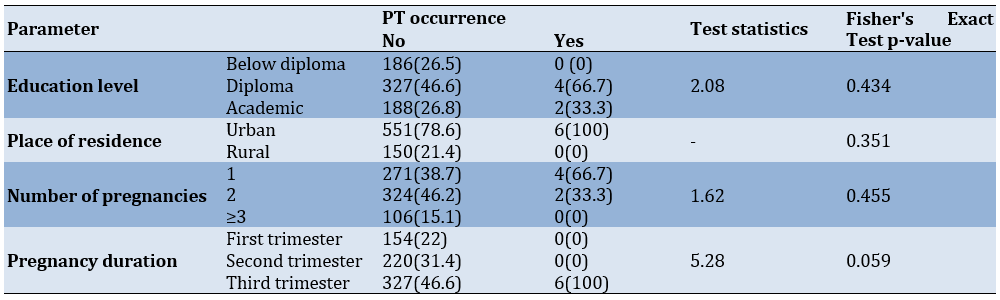
There was no significant difference in the incidence of PTs concerning demographic information and the factors examined (p-value>0.05 for all cases). However, the frequency of PT was higher in the third trimester of pregnancy compared to other times (Table 1).
Table 1. Frequency of patients by pregnancy tumor occurrence according to demographic information, medical history, pregnancy trimester, and intervals between pregnancies
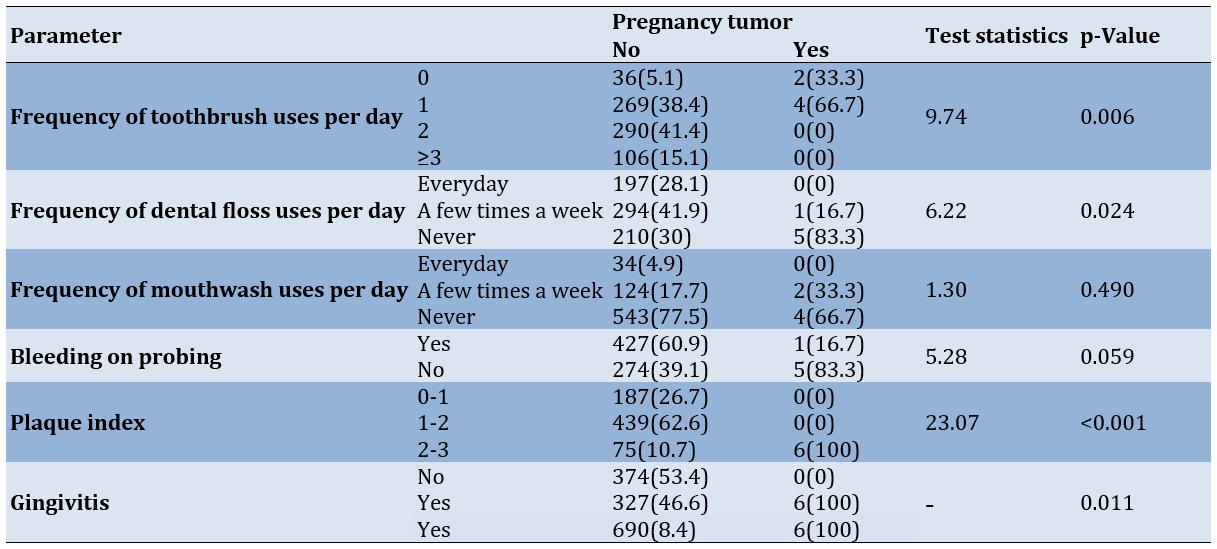
The results showed significant differences between the groups (with and without PT) in terms of the frequency of toothbrush use (p-value=0.006), dental floss use (p-value=0.024), the presence of gingivitis (p-value=0.011), and PI categories (p-value=0.006). The frequency of PT was higher among individuals who brushed and flossed less frequently each day, had positive BOP, higher PI (2 to 3), and greater gingival inflammation. Additionally, the mean PI (2.26±0.63) and the mean number of quadrants affected by gingival inflammation (2.33±0.52) were significantly higher in individuals with PT compared to those without, who had a mean PI of 1.41 ± 0.49 and a mean number of affected quadrants of 0.73±0.94 (both with p-value<0.001). However, no significant differences were observed between the two groups regarding the frequency of mouthwash use per day (p-value=0.490) and the presence of inappropriate crowns/restorations (p-value=0.999; Table 2).
Table 2. Frequency of patients regarding the incidence of pregnancy tumors according to oral hygiene status and related indices and parameters
Discussion
This study assessed the frequency of PTs among pregnant women referred to Al-Zahra Hospital in Rasht, Iran, during 2022-2023. This rate was found to be 0.8%. Similar findings were reported by Tabatabaei Nejad et al. [7], with a prevalence of 0.22% (2 out of 923), and Kia et al. [13], with a prevalence of 0.67% (2 out of 300). Other studies, including those by Khatibi et al. [3], Molania et al. [4], and Chamani et al. [8], found higher prevalences of 4.5%, 4.38%, and 4.2%, respectively. Additionally, Saebi & Robati reported a PT prevalence of 10% [14].
The discrepancies in prevalence rates could be attributed to differences in cultural and health conditions among the populations studied, as well as variations in diagnostic methods (clinical vs. histopathological). In our study and that of Tabatabaei Nejad et al. [7], clinical examinations were supplemented with biopsies and histopathological analyses to confirm PT lesions, thereby increasing diagnostic accuracy compared to studies that relied solely on clinical examinations, such as that by Chamani et al. [8]. The high prevalence reported by Saebi & Robati (10%) may result from data collection based only on patient records without clinical examinations [14]. Hormonal changes during pregnancy, including increased levels of estrogen, progesterone, and placental gonadotropin, can lead to vascular changes such as increased permeability and endothelial cell swelling. These changes, along with alterations in the oral microbiome, decreased keratinization of the gingival epithelium, and immunological changes, may increase susceptibility to PT lesions [2].
In this study, all observed PT lesions were single, swollen, red lesions measuring 5mm or less, located in the interproximal papilla of the mandibular gums, with no reported pain. Previous research indicates that PT and PG lesions are more common in the maxillary gums and generally exhibit a slow clinical progression. These lesions often present as asymptomatic pedunculated or sessile protrusions but can occasionally cause bleeding, pain, or tenderness [2, 15, 16]. The higher prevalence of PT in the mandibular gums observed in this study may be attributed to the higher levels of plaque and tartar in the lower jaw, as poor oral hygiene and dental plaque are significant factors in the development of PG/PT [2].
The overall mean age of participants was 28.60±4.77 years, with the PT group averaging 30.00 ± 4.98 years. In other studies by Khatibi et al., Molania et al., and Chamani et al., mean ages of 26.8, 26.9, and 28.9 years are reported, respectively [3, 4, 8]. Our study found no significant relationship between age and PT prevalence, which contrasts with Khatibi et al., who reported a higher prevalence in individuals over 25 years old [3]. However, other studies did not find a significant relationship between age and PT prevalence, which aligns with our findings [4, 8].
In terms of education, 26.5% of participants had less than a diploma, 46.6% had a diploma, and 26.6% had a university education. Among PT patients, four cases had a diploma, and two cases had a university education, with no significant relationship found between education level and PT incidence.
Of the participants, 78.6% reported living in urban areas, while 21.4% were living in rural areas, with all PT patients residing in urban areas. No significant relationship was found between residence and PT incidence. Only Al-Noaman reports a higher prevalence of PG among rural residents [17]. Given the reported correlations between education, socioeconomic status (income), and awareness of oral hygiene among pregnant women [17, 18], further studies with larger sample sizes are recommended to investigate the relationship between residence, education, and PT prevalence more thoroughly.
There was a significant relationship between the prevalence of PTs and poor oral hygiene among the study participants. Most participants brushed their teeth twice a day (41.4%) and flossed several times a week (41.9%). However, the prevalence of PT was higher among those who did not brush their teeth at all (66.7%) or brushed only once a day (33.3%), as well as among those who did not use dental floss (83.3%). Conversely, there was no significant relationship between mouthwash use and PT, as over two-thirds of all participants (77.5%) and more than half of those with PT (66.7%) did not use mouthwash. These findings align with the studies by Tabatabaei Nejad et al. and Chamani et al., reporting poor oral hygiene or heavy dental calculus in PT patients but finding no significant relationship between mouthwash use and PT prevalence [7, 8]. Khatibi et al. similarly found a significant relationship between PT prevalence and poor oral hygiene, reporting that PT patients are 2.5 times more likely to have poor oral hygiene compared to those without PT [3]. According to Chamani et al., there is a correlation between the frequency of flossing and PT prevalence [8].
By comparing demographic information (age, education level, and place of residence) with oral hygiene indices, it is evident that poor oral hygiene is a more significant factor in the occurrence of PTs. Additionally, since 66.7% of PT patients had non-university education, lower education levels and, consequently, lower awareness of oral hygiene practices could be considered indirect influencing factors in PT incidence.
In this study, approximately 66% of PT patients (four out of six) were in their first pregnancy, and about 33% (two out of six) were in their second pregnancy, with no significant relationship found between PT incidence and pregnancy history. All six PT patients were in their third trimester, but there was no significant relationship between PT incidence and the trimester of pregnancy. While PT is often reported to occur early in the third trimester, primarily due to hormonal changes during this period [4, 15], according to Khatibi et al., a higher prevalence of PT is observed in the third trimester. The discrepancy in the present study could be due to the small number of PT cases and the fact that more pregnant women visit Al-Zahra Hospital in late pregnancy than in early pregnancy.
Gingivitis during pregnancy has two peak periods, including the first in the first trimester, coinciding with high levels of gonadotropins, and the second in the third trimester, related to peak levels of estrogen and progesterone [19]. Therefore, the onset of PT in the first trimester and its increased prevalence in the third trimester is predictable. While hormonal and vascular changes in pregnant women can enhance the inflammatory response of oral tissues, including the gums, to factors, such as dental calculus and plaque that promote PT [8], it has been reported that maintaining proper oral hygiene can mitigate the impact of hormonal changes on PT occurrence [20].
In this study, the number of participants with negative BOP (60.9%) was approximately 1.5 times higher than those with positive BOP (39.1%). Additionally, most of the participants with PT had positive BOP (5 out of 6), indicating a significantly higher prevalence of PT among pregnant women with positive BOP. Consistent with our findings, studies by Alnasser et al. and Oliveira et al. also reported an increase in pocket depth and BOP as clinical indicators of periodontal diseases in pregnant women [21, 22]. According to Gürsoy et al., there is an increase in BOP without an association with dental plaque from the first to the second trimester, while no change in BOP is observed in the non-pregnant group [23].
Among all participants, 26.7% had a PI between zero and one, 62.6% had a PI between one and two, and 10.7% had a PI between two and three. All PT patients in this study had a PI between two and three. The prevalence of PT was significantly higher among participants with a PI of two to three. The mean PI in PT patients (2.26) was about 1.5 times higher than that in non-PT individuals (1.41). Molania et al. also found a significant association between infrequent tooth brushing and flossing and the prevalence of PT/PG [4]. Gil et al. reported an increase in BOP during pregnancy and a significant relationship between mean PI, the frequency of tooth brushing, and the severity of periodontal diseases in pregnant women [24]. Shamsi et al. also report a significant increase in PI among pregnant women compared to the control group [25]. Although our study did not find a statistically significant relationship between PT prevalence and positive BOP, positive BOP was more common in PT patients. Gil et al. also highlight the increase in BOP during pregnancy [24]. Given the small number of PT cases in this study, further research in this area is warranted.
Less than half of the participants (46.6%) had gingivitis. There was a significant relationship between gingivitis and PT prevalence, with a higher frequency of PT among those with gingivitis. Islam & Haque also note an increase in gingivitis and periodontal diseases during pregnancy [26]. The presence of bacteria, such as Porphyromonas gingivalis has been reported to increase gingivitis during pregnancy [27]. Aoun and Ojanotko-Harri et al. describe the role of estrogen in increasing vascular endothelial growth factor production in macrophages and the role of progesterone as an immunosuppressant in the gums, reducing acute inflammatory responses, and increasing chronic proliferative reactions to bacteria [28, 29]. Therefore, the increase in these hormones during pregnancy, combined with the presence of bacteria, may enhance susceptibility to PT.
The comprehensive examination of the relationship between periodontal factors, such as PI, BOP, and gingivitis with PT or PG in Iran is a strength of this study. However, the small number of PT cases in the sample limits the generalizability of some results, suggesting the need for larger studies in the future. Given the significant relationship between poor oral hygiene, particularly the lack of tooth brushing and flossing, and higher dental plaque indices, it is recommended that women planning to conceive be referred to dentists for necessary preventive and dental care measures more rigorously.
Conclusion
The prevalence of PTs among pregnant women is 0.8%.
Acknowledgments: We extend our sincere thanks to Gilan University of Medical Sciences for supporting this research project and to Dr. Mohammad Ebrahim Ghafari, an Associate Professor of Biostatistics, for performing the statistical analysis throughout the paper.
Ethical Permissions: This study was approved by the Ethics Committee of Gilan University of Medical Sciences (IR.GUMS.REC.1401.529) and received cooperation consent from Al-Zahra Hospital in Rasht.
Authors' Contribution: Samami M (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (30%); Salari A (Second Author), Introduction Writer/Assistant Researcher/Statistical Analyst (20%); Sharami SH (Third Author), Introduction Writer/Assistant Researcher/Discussion Writer/Statistical Analyst (20%); Jafari Sadatmahalle Z (Fourth Author), Introduction Writer/Discussion Writer (15%); Jafari Najar-Karimi F (Fifth Author), Introduction Writer/Discussion Writer (15%)
Funding/Support: This work was supported by the Guilan University of Medical Sciences.
The rising incidence of oral and dental diseases is a major concern for pregnant women, as it adversely affects their health and quality of life and poses risks for the fetus, including low birth weight [1]. Pyogenic granuloma (PG) is a benign vascular lesion that often grows rapidly and tends to bleed, predominantly found in the oral cavity, especially on the gums [2]. A specific type of PG, known as pregnancy tumor (PT) or granuloma gravidarum, commonly develops in pregnant women between the third and ninth months of pregnancy. Its size often increases toward the end of pregnancy due to hormonal changes, particularly elevated estrogen levels [3, 4]. Clinically, PT appears as an exophytic lesion with a smooth or lobulated surface, often ulcerated and reddish, usually pedunculated, and ranges in size from a few millimeters to up to 2 centimeters. It bleeds easily, even with minor irritation, such as brushing teeth. Although it can occur anywhere in the oral cavity, it is more prevalent on the upper gum, leading to secondary complications due to challenges in maintaining oral hygiene because of excessive bleeding. These complications include increased pocket depth, difficulty in removing microbial plaque, higher rates of dental caries, periodontal diseases, and an elevated risk of oral infections [2, 3].
Since this lesion is reactive and exacerbated by hormonal changes during pregnancy, such as increased estrogen activity on vascular tissues, along with chronic local irritants like tartar, dental plaque, broken and defective restorations, sharp teeth, and poor oral hygiene, it can be prevented by maintaining good oral hygiene and undergoing prophylactic treatments during pregnancy [2, 3].
If the PT is large, it can interfere with eating or brushing the teeth of pregnant women and cause severe discomfort due to bleeding and pain, which can lead to insufficient nutrition for the mother and inadequate development of the fetus, necessitating surgical removal. However, if patients are aware of how to prevent or control this lesion and if the size of the PT is small, it may heal spontaneously due to the sudden drop in estrogen levels after delivery [5, 6]. Therefore, it is essential to know the prevalence of this lesion in order to plan for its prevention and control of the aggravating factors.
Given the significant cultural differences in oral hygiene practices across various communities and the need to understand the prevalence of PT lesions for planning appropriate preventive measures in oral health education programs for pregnant women, it is essential to determine the prevalence of these lesions in the target population. Although various studies have examined the prevalence of these lesions [3, 4, 7], there is no consensus on the reported percentages across different communities. Due to the scarcity and outdated nature of studies and the lack of recent statistics in Gilan province, this study aimed to investigate the frequency of PTs and associated factors among pregnant women attending Al-Zahra Hospital in Rasht.
Instrument and Methods
Design
This cross-sectional analytical study included pregnant women who visited Al-Zahra Hospital in Rasht during the first, second, or third trimesters from 2022 to 2023, without any limitations on age, occupation, or other factors. The participants’ pregnancies were confirmed through urine HCG tests, supplementary blood tests, and ultrasounds.
Inclusion and exclusion criteria
Individuals with hormonal abnormalities, such as hyperthyroidism, specific infectious or systemic diseases, like HIV or diabetes, a history of malignancies, or concurrent tooth mobility or suspicious symptoms, like paresthesia alongside the exophytic lesion were excluded from the study. Any red to pink, pedunculated, or non-pedunculated lesions with soft to firm consistency that developed on the gums at any time during pregnancy were considered suspicious for PTs and included in the study [8]. The diagnosis of PTs was based on clinical appearance and histopathological examination.
Sampling
Sampling was conducted using simple random sampling. Samples were selected based on the last three digits of the medical records of patients visiting Al-Zahra Hospital, utilizing a random number table. The required sample size, based on the main objective and the estimated prevalence from the study by Patil [9], considering α=0.05 and d=0.02, was at least 707.
Data collection
Each patient completed a form with basic individual, demographic, and clinical information, including age, education level (below diploma/diploma/university degree), place of residence (urban/rural), medical and dental history, pregnancy duration (first, second, or third trimester), number of pregnancies, and the interval between pregnancies (in years).
Clinical examinations were performed using a “Williams” graduated probe and a disposable dental mirror (Brilliant model, Teksan Co., Iran) under adequate lighting. The buccal and lingual gums of the upper and lower jaws were examined by a final-year dental student under the supervision of an oral and maxillofacial specialist. Observations were recorded on a pre-prepared checklist by the researcher, which included the presence and location of the lesion, gingival inflammation (present/absent), the average number of quadrants with gingival inflammation, the bleeding on probing (BOP) index (positive/negative), plaque index (PI; ranging from 0 to 3 and average PI), frequency of daily use of a toothbrush, floss, and mouthwash, and the presence of inappropriate dental crowns/restorations in the lesion area.
After a thorough clinical examination of the intraoral and extraoral mucosa, any suspicious PTs identified were biopsied for further examination by an oral and maxillofacial specialist. The biopsied samples, after histopathological review and confirmation of PG by an oral pathologist, were included in the study. The BOP index was used to assess active gingivitis, evaluated based on clinical bleeding from the gingival margin using a Williams probe with appropriate pressure (0.75N, approximately 20-25g) and observing for bleeding within 10 seconds, ensuring that probing depth did not exceed 3mm at any point in the mouth. The Silness & Loe PI was also utilized [10-12].
Data analysis
Descriptive statistics for qualitative data were reported as frequencies and percentages, while quantitative data were presented as means and standard deviations. The Kolmogorov-Smirnov test was used to check the normality of the distribution of the groups. Depending on the type of parameter, the independent t-test and Chi-square test were used to compare groups if the assumptions were met; otherwise, Mann-Whitney U and Fisher’s exact tests were employed. Logistic regression analysis was used to simultaneously examine independent parameters and adjust for potential confounding factors. Data were analyzed using SPSS 28, at a significance level of p-value≤0.05.
Findings
Among the 707 pregnant women examined, with a mean age of 29.30±4.87 years, six cases (0.8%) had PT lesions (mean age=30.00±4.98 years), while 701 women (98.2%) were without lesions (mean age=28.60±4.77 years).
The average age of the group with PT was 30.00±4.98 years, and the average age of the group without PT was 28.60±4.77 years, with no significant difference between the two groups (p-value=0.473). Additionally, the average interval between pregnancies for the group with PT was 2.67±4.13 years, while for the group without PT, it was 30.00±4.98 years, and there was no significant difference between the two groups (p-value=0.376).
There was no significant difference in the incidence of PTs concerning demographic information and the factors examined (p-value>0.05 for all cases). However, the frequency of PT was higher in the third trimester of pregnancy compared to other times (Table 1).
Table 1. Frequency of patients by pregnancy tumor occurrence according to demographic information, medical history, pregnancy trimester, and intervals between pregnancies

The results showed significant differences between the groups (with and without PT) in terms of the frequency of toothbrush use (p-value=0.006), dental floss use (p-value=0.024), the presence of gingivitis (p-value=0.011), and PI categories (p-value=0.006). The frequency of PT was higher among individuals who brushed and flossed less frequently each day, had positive BOP, higher PI (2 to 3), and greater gingival inflammation. Additionally, the mean PI (2.26±0.63) and the mean number of quadrants affected by gingival inflammation (2.33±0.52) were significantly higher in individuals with PT compared to those without, who had a mean PI of 1.41 ± 0.49 and a mean number of affected quadrants of 0.73±0.94 (both with p-value<0.001). However, no significant differences were observed between the two groups regarding the frequency of mouthwash use per day (p-value=0.490) and the presence of inappropriate crowns/restorations (p-value=0.999; Table 2).
Table 2. Frequency of patients regarding the incidence of pregnancy tumors according to oral hygiene status and related indices and parameters

Discussion
This study assessed the frequency of PTs among pregnant women referred to Al-Zahra Hospital in Rasht, Iran, during 2022-2023. This rate was found to be 0.8%. Similar findings were reported by Tabatabaei Nejad et al. [7], with a prevalence of 0.22% (2 out of 923), and Kia et al. [13], with a prevalence of 0.67% (2 out of 300). Other studies, including those by Khatibi et al. [3], Molania et al. [4], and Chamani et al. [8], found higher prevalences of 4.5%, 4.38%, and 4.2%, respectively. Additionally, Saebi & Robati reported a PT prevalence of 10% [14].
The discrepancies in prevalence rates could be attributed to differences in cultural and health conditions among the populations studied, as well as variations in diagnostic methods (clinical vs. histopathological). In our study and that of Tabatabaei Nejad et al. [7], clinical examinations were supplemented with biopsies and histopathological analyses to confirm PT lesions, thereby increasing diagnostic accuracy compared to studies that relied solely on clinical examinations, such as that by Chamani et al. [8]. The high prevalence reported by Saebi & Robati (10%) may result from data collection based only on patient records without clinical examinations [14]. Hormonal changes during pregnancy, including increased levels of estrogen, progesterone, and placental gonadotropin, can lead to vascular changes such as increased permeability and endothelial cell swelling. These changes, along with alterations in the oral microbiome, decreased keratinization of the gingival epithelium, and immunological changes, may increase susceptibility to PT lesions [2].
In this study, all observed PT lesions were single, swollen, red lesions measuring 5mm or less, located in the interproximal papilla of the mandibular gums, with no reported pain. Previous research indicates that PT and PG lesions are more common in the maxillary gums and generally exhibit a slow clinical progression. These lesions often present as asymptomatic pedunculated or sessile protrusions but can occasionally cause bleeding, pain, or tenderness [2, 15, 16]. The higher prevalence of PT in the mandibular gums observed in this study may be attributed to the higher levels of plaque and tartar in the lower jaw, as poor oral hygiene and dental plaque are significant factors in the development of PG/PT [2].
The overall mean age of participants was 28.60±4.77 years, with the PT group averaging 30.00 ± 4.98 years. In other studies by Khatibi et al., Molania et al., and Chamani et al., mean ages of 26.8, 26.9, and 28.9 years are reported, respectively [3, 4, 8]. Our study found no significant relationship between age and PT prevalence, which contrasts with Khatibi et al., who reported a higher prevalence in individuals over 25 years old [3]. However, other studies did not find a significant relationship between age and PT prevalence, which aligns with our findings [4, 8].
In terms of education, 26.5% of participants had less than a diploma, 46.6% had a diploma, and 26.6% had a university education. Among PT patients, four cases had a diploma, and two cases had a university education, with no significant relationship found between education level and PT incidence.
Of the participants, 78.6% reported living in urban areas, while 21.4% were living in rural areas, with all PT patients residing in urban areas. No significant relationship was found between residence and PT incidence. Only Al-Noaman reports a higher prevalence of PG among rural residents [17]. Given the reported correlations between education, socioeconomic status (income), and awareness of oral hygiene among pregnant women [17, 18], further studies with larger sample sizes are recommended to investigate the relationship between residence, education, and PT prevalence more thoroughly.
There was a significant relationship between the prevalence of PTs and poor oral hygiene among the study participants. Most participants brushed their teeth twice a day (41.4%) and flossed several times a week (41.9%). However, the prevalence of PT was higher among those who did not brush their teeth at all (66.7%) or brushed only once a day (33.3%), as well as among those who did not use dental floss (83.3%). Conversely, there was no significant relationship between mouthwash use and PT, as over two-thirds of all participants (77.5%) and more than half of those with PT (66.7%) did not use mouthwash. These findings align with the studies by Tabatabaei Nejad et al. and Chamani et al., reporting poor oral hygiene or heavy dental calculus in PT patients but finding no significant relationship between mouthwash use and PT prevalence [7, 8]. Khatibi et al. similarly found a significant relationship between PT prevalence and poor oral hygiene, reporting that PT patients are 2.5 times more likely to have poor oral hygiene compared to those without PT [3]. According to Chamani et al., there is a correlation between the frequency of flossing and PT prevalence [8].
By comparing demographic information (age, education level, and place of residence) with oral hygiene indices, it is evident that poor oral hygiene is a more significant factor in the occurrence of PTs. Additionally, since 66.7% of PT patients had non-university education, lower education levels and, consequently, lower awareness of oral hygiene practices could be considered indirect influencing factors in PT incidence.
In this study, approximately 66% of PT patients (four out of six) were in their first pregnancy, and about 33% (two out of six) were in their second pregnancy, with no significant relationship found between PT incidence and pregnancy history. All six PT patients were in their third trimester, but there was no significant relationship between PT incidence and the trimester of pregnancy. While PT is often reported to occur early in the third trimester, primarily due to hormonal changes during this period [4, 15], according to Khatibi et al., a higher prevalence of PT is observed in the third trimester. The discrepancy in the present study could be due to the small number of PT cases and the fact that more pregnant women visit Al-Zahra Hospital in late pregnancy than in early pregnancy.
Gingivitis during pregnancy has two peak periods, including the first in the first trimester, coinciding with high levels of gonadotropins, and the second in the third trimester, related to peak levels of estrogen and progesterone [19]. Therefore, the onset of PT in the first trimester and its increased prevalence in the third trimester is predictable. While hormonal and vascular changes in pregnant women can enhance the inflammatory response of oral tissues, including the gums, to factors, such as dental calculus and plaque that promote PT [8], it has been reported that maintaining proper oral hygiene can mitigate the impact of hormonal changes on PT occurrence [20].
In this study, the number of participants with negative BOP (60.9%) was approximately 1.5 times higher than those with positive BOP (39.1%). Additionally, most of the participants with PT had positive BOP (5 out of 6), indicating a significantly higher prevalence of PT among pregnant women with positive BOP. Consistent with our findings, studies by Alnasser et al. and Oliveira et al. also reported an increase in pocket depth and BOP as clinical indicators of periodontal diseases in pregnant women [21, 22]. According to Gürsoy et al., there is an increase in BOP without an association with dental plaque from the first to the second trimester, while no change in BOP is observed in the non-pregnant group [23].
Among all participants, 26.7% had a PI between zero and one, 62.6% had a PI between one and two, and 10.7% had a PI between two and three. All PT patients in this study had a PI between two and three. The prevalence of PT was significantly higher among participants with a PI of two to three. The mean PI in PT patients (2.26) was about 1.5 times higher than that in non-PT individuals (1.41). Molania et al. also found a significant association between infrequent tooth brushing and flossing and the prevalence of PT/PG [4]. Gil et al. reported an increase in BOP during pregnancy and a significant relationship between mean PI, the frequency of tooth brushing, and the severity of periodontal diseases in pregnant women [24]. Shamsi et al. also report a significant increase in PI among pregnant women compared to the control group [25]. Although our study did not find a statistically significant relationship between PT prevalence and positive BOP, positive BOP was more common in PT patients. Gil et al. also highlight the increase in BOP during pregnancy [24]. Given the small number of PT cases in this study, further research in this area is warranted.
Less than half of the participants (46.6%) had gingivitis. There was a significant relationship between gingivitis and PT prevalence, with a higher frequency of PT among those with gingivitis. Islam & Haque also note an increase in gingivitis and periodontal diseases during pregnancy [26]. The presence of bacteria, such as Porphyromonas gingivalis has been reported to increase gingivitis during pregnancy [27]. Aoun and Ojanotko-Harri et al. describe the role of estrogen in increasing vascular endothelial growth factor production in macrophages and the role of progesterone as an immunosuppressant in the gums, reducing acute inflammatory responses, and increasing chronic proliferative reactions to bacteria [28, 29]. Therefore, the increase in these hormones during pregnancy, combined with the presence of bacteria, may enhance susceptibility to PT.
The comprehensive examination of the relationship between periodontal factors, such as PI, BOP, and gingivitis with PT or PG in Iran is a strength of this study. However, the small number of PT cases in the sample limits the generalizability of some results, suggesting the need for larger studies in the future. Given the significant relationship between poor oral hygiene, particularly the lack of tooth brushing and flossing, and higher dental plaque indices, it is recommended that women planning to conceive be referred to dentists for necessary preventive and dental care measures more rigorously.
Conclusion
The prevalence of PTs among pregnant women is 0.8%.
Acknowledgments: We extend our sincere thanks to Gilan University of Medical Sciences for supporting this research project and to Dr. Mohammad Ebrahim Ghafari, an Associate Professor of Biostatistics, for performing the statistical analysis throughout the paper.
Ethical Permissions: This study was approved by the Ethics Committee of Gilan University of Medical Sciences (IR.GUMS.REC.1401.529) and received cooperation consent from Al-Zahra Hospital in Rasht.
Authors' Contribution: Samami M (First Author), Introduction Writer/Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (30%); Salari A (Second Author), Introduction Writer/Assistant Researcher/Statistical Analyst (20%); Sharami SH (Third Author), Introduction Writer/Assistant Researcher/Discussion Writer/Statistical Analyst (20%); Jafari Sadatmahalle Z (Fourth Author), Introduction Writer/Discussion Writer (15%); Jafari Najar-Karimi F (Fifth Author), Introduction Writer/Discussion Writer (15%)
Funding/Support: This work was supported by the Guilan University of Medical Sciences.
Article Type: Descriptive & Survey |
Subject:
Oral Health Education/Promotion
Received: 2024/06/23 | Accepted: 2024/09/29 | Published: 2024/10/8
Received: 2024/06/23 | Accepted: 2024/09/29 | Published: 2024/10/8
References
1. Hong HH, Chen YH, Cheng PJ, Chang MY, Chuang LL. Risk factors associated with periodontal disease and its impact on quality of life among pregnant women. J Obstet Gynaecol. 2023;43(2):2264382. [Link] [DOI:10.1080/01443615.2023.2264382]
2. Lomeli Martinez SM, Carrillo Contreras NG, Gómez Sandoval JR, Zepeda Nuño JS, Gomez Mireles JC, Varela Hernández JJ, et al. Oral pyogenic granuloma: A narrative review. Int J Mol Sci. 2023;24(23):16885. [Link] [DOI:10.3390/ijms242316885]
3. Khatibi M, Niromanesh S, Abhari SY, Falakaflaki N. Prevalence of pregnancy tumor (pyogenic granuloma) and related factors in pregnant women referred to Tehran Mirza Kuchak Khan Hospital during 2010-2011. Iran J Obstet Gynecol Infertil. 2013;16(71):1-6. [Persian] [Link]
4. Molania T, Salehabadi N, Zahedpasha S, Yazdani Charati J, Imani B, Ghasemi S, et al. Frequency of epulis gravidarum in pregnant. J Nurs Midwifery Sci. 2022;9(4):303-9. [Link]
5. Radwan-Oczko M, Hirnle L, Szczepaniak M, Duś-Ilnicka I. How much do pregnant women know about the importance of oral health in pregnancy? Questionnaire-based survey. BMC Pregnancy Childbirth. 2023;23(1):348. [Link] [DOI:10.1186/s12884-023-05677-4]
6. Sharma A, Mathur VP, Sardana D. Effective management of a pregnancy tumour using a soft tissue diode laser: A case report. Laser Ther. 2014;23(4):279-82. [Link] [DOI:10.5978/islsm.14-CR-02]
7. Tabatabaei Nejad ES, BigomTaheri J, Azimi S. Frequency of gingival pregnancy tumor in iran (confirmed by biopsy). J Int Oral Health. 2014;6(6):72-6. [Link]
8. Chamani G, Navabi N, Abdollahzadeh S. Prevalence of pregnancy tumor in pregnant women. J Dent. 2009;10(1):79-82. [Persian] [Link]
9. Patil S. Oral changes in pregnant and nonpregnant women: A case-control study. J Orofac Sci. 2013;5(2):118-22. [Link] [DOI:10.4103/0975-8844.124257]
10. Gil-Montoya JA, Rivero-Blanco T, Leon-Rios X, Exposito-Ruiz M, Pérez-Castillo I, Aguilar-Cordero MJ. Oral and general health conditions involved in periodontal status during pregnancy: A prospective cohort study. Arch Gynecol Obstet. 2023;308(6):1765-73. [Link] [DOI:10.1007/s00404-022-06843-3]
11. Ayobiyan N, Afsar N. The effect of aspirin on bleeding on probing in patients with gingivitis. J Dent. 2009;10(4):337-42. [Persian] [Link]
12. Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121-35. [Link] [DOI:10.3109/00016356408993968]
13. Kia SJ, Vadiati Saberi B, Moonesan MR, Madani AR. Frequency of intra and extra oral manifestations of pregnant women in Rasht (2013). J Inflamm Dis. 2017;20(6):e155997. [Persian] [Link]
14. Saebi KH, Robati B. Evaluation of periodontium tissue in pregnancy period in referred patient to Mashhad dental University. J Mashhad Univ Med Sci Dent Coll. 1993;14:165-71. [Persian] [Link]
15. Meshram M, Durge K, Shirbhate U. An overview of oral pyogenic granuloma and its management: A case report. Cureus. 2023;15(11):e48305. [Link] [DOI:10.7759/cureus.48305]
16. Sonar PR, Panchbhai AS. Pyogenic granuloma in the mandibular anterior gingiva: A case study. Cureus. 2024;16(1):e52273. [Link] [DOI:10.7759/cureus.52273]
17. Al-Noaman AS. Pyogenic granuloma: Clinicopathological and treatment scenario. J Indian Soc Periodontol. 2020;24(3):233-6. [Link] [DOI:10.4103/jisp.jisp_132_19]
18. Flores DAF, López ENC. Relationship between oral health and nutrition during pregnancy, nursing, and the first two years of life. In: The role of nutrition in integral health and quality of life. Canada: Apple Academic Press; 2024. p. 409-54. [Link] [DOI:10.1201/9781003413585-18]
19. Kashetty M, Kumbhar S, Patil S, Patil P. Oral hygiene status, gingival status, periodontal status, and treatment needs among pregnant and nonpregnant women: A comparative study. J Indian Soc Periodontol. 2018;22(2):164-70. [Link] [DOI:10.4103/jisp.jisp_319_17]
20. Wu M, Chen SW, Su WL, Zhu HY, Ouyang SY, Cao YT, et al. Sex hormones enhance gingival inflammation without affecting IL-1β and TNF-α in periodontally healthy women during pregnancy. Mediators Inflamm. 2016;2016:4897890. [Link] [DOI:10.1155/2016/4897890]
21. Alnasser BH, Alkhaldi NK, Alghamdi WK, Alghamdi FT. The potential association between periodontal diseases and adverse pregnancy outcomes in pregnant women: A systematic review of randomized clinical trials. Cureus. 2023;15(1):e33216. [Link] [DOI:10.7759/cureus.33216]
22. Oliveira LJC, Cademartori MG, Sfreddo CS, Silveira MF, Barros FC, Correa MB, et al. Factors associated with periodontal diseases in pregnancy: Findings of the 2015 pelotas birth cohort study. Braz Oral Res. 2023;37:e110. [Link] [DOI:10.1590/1807-3107bor-2023.vol37.0110]
23. Gürsoy M, Pajukanta R, Sorsa T, Könönen E. Clinical changes in periodontium during pregnancy and post-partum. J Clin Periodontol. 2008;35(7):576-83. [Link] [DOI:10.1111/j.1600-051X.2008.01236.x]
24. Gil L, Mínguez I, Caffesse R, Llambés F. Periodontal disease in pregnancy: The influence of general factors and inflammatory mediators. Oral Health Prev Dent. 2019;17(1):69-73. [Link]
25. Shamsi M, Hidarnia A, Niknami S, karimi M. Effects of educational programs on DMFT plaque index and performance of pregnant women. J Mazandaran Univ Med Sci. 2013;23(100):62-72. [Persian] [Link]
26. Islam NAB, Haque A. Pregnancy-related dental problems: A review. Heliyon. 2024;10(3):e24259. [Link] [DOI:10.1016/j.heliyon.2024.e24259]
27. Carrillo-De-Albornoz A, Figuero E, Herrera D, Cuesta P, Bascones-Martínez A. Gingival changes during pregnancy: III. Impact of clinical, microbiological, immunological and socio-demographic factors on gingival inflammation. J Clin Periodontol. 2012;39(3):272-83. [Link] [DOI:10.1111/j.1600-051X.2011.01800.x]
28. Ojanotko‐Harri AO, Harri MP, Hurttia HM, Sewoón LA. Altered tissue metabolism of progesterone in pregnancy gingivitis and granuloma. J Clin Periodontol. 1991;18(4):262-6. [Link] [DOI:10.1111/j.1600-051X.1991.tb00425.x]
29. Aoun G. Pyogenic granuloma of the oral cavity: Clinical and histopathological features, etiopathogenesis, and management. J Health Sci Res. 2023;8(1):26-8. [Link] [DOI:10.7324/jhsr.2023.815]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






