Volume 11, Issue 3 (2023)
Health Educ Health Promot 2023, 11(3): 341-348 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Yousif M, Al-Jumeily D, Al-Amran F, Sadeq A, Rawaf S. Effect of COVID-19 Vaccines on Hair Loss. Health Educ Health Promot 2023; 11 (3) :341-348
URL: http://hehp.modares.ac.ir/article-5-68676-en.html
URL: http://hehp.modares.ac.ir/article-5-68676-en.html
1- Biology Department, College of Science, University of Al-Qadisiyah, Al-Qadisiyah, Iraq
2- Department of Artificial Intelligence, Faculty of Engineering and Technology, John Moores University, Liverpool, UK
3- Cardiovascular Department, College of Medicine, Kufa University, Al-Najaf, Iraq
4- Department of Obstetrics and Gynecology, College of Medicine, Kufa University, Al-Najaf, Iraq
5- WHO Collaboration Center, Imperial College, London, UK
2- Department of Artificial Intelligence, Faculty of Engineering and Technology, John Moores University, Liverpool, UK
3- Cardiovascular Department, College of Medicine, Kufa University, Al-Najaf, Iraq
4- Department of Obstetrics and Gynecology, College of Medicine, Kufa University, Al-Najaf, Iraq
5- WHO Collaboration Center, Imperial College, London, UK
Keywords: COVID-19 Vaccines [MeSH], Hair Loss [MeSH], Machine Learning [MeSH], Sociodemographic Factors [MeSH], Comorbidity [MeSH]
Full-Text [PDF 841 kb]
(4153 Downloads)
| Abstract (HTML) (1810 Views)
Full-Text: (902 Views)
Introduction
The novel coronavirus disease 2019 (COVID-19) outbreak was declared a global pandemic by the World Health Organization (WHO) on March 11, 2020 [1]. Over 468 million infections and more than 6 million deaths worldwide have been caused by the Coronavirus disease 2019 (COVID-19) [2]. Various vaccines have been developed and approved for COVID-19 globally. Due to the new variants (e.g., Omicron), the use of additional doses is also being reviewed and approved for prevention [3]. The COVID-19 pandemic has had a severe effect on world health and the economy, and vaccination has been critical in limiting its spread [4, 5].
There is still insufficient information on the immune-related issues of COVID-19 vaccines and whether or not they can cause the flare of autoimmune diseases [6]. The quick development and distribution of COVID-19 vaccination has aided in the reduction of cases and deaths globally [7]. However, while the efficacy and safety of vaccines have been extensively studied, their potential side effects remain a concern [8].
Vaccine-induced adverse events are a concern among both healthy individuals and those with preexisting conditions. The concern is that the safety of any vaccine cannot be easily generalizable to populations who are not routinely included in clinical trials, such as patients with chronic autoimmune and inflammatory disorders [9]. The paucity of data and concerns related to disease exacerbations of these preexisting inflammatory conditions can serve as a source of vaccine hesitancy among patients. Several studies have observed exacerbations of preexisting autoimmune or autoinflammatory disorders in at-risk populations postvaccination [10].
The development of vaccines throughout human history has changed the course of diseases and humanity [11]. The process of vaccine development often requires decades to obtain a marketable vaccine; nevertheless, with the advent of the SARS-CoV-2 pandemic in March 2020, the process was accelerated by both necessity and the amount of resources available for this purpose [12]. While this process has followed the highest standards of safety and control, due to the large number of people vaccinated in all corners of the globe, reports of adverse events are still very common [13, 14]. In this context, by December 2020, the Food and Drug Administration (FDA) authorized the emerging use of the mRNA-based vaccine produced by Pfizer/BioNTech (BNT162b2), followed by Moderna (ARNm-1273), and later the rest of the COVID-19 vaccines, including the viral-vector-based AstraZeneca (ChAdOx1), or the attenuated and inactivated virus vaccine from Sinopharm (BBIBP-CorV) and Sinovac (Sinovac-CoronaVac) [15].
With initial reports on safety and effectivity, and after the biggest vaccination campaign took place all over the planet, adverse events started to appear [15, 16]. Among the most common and expected are pain at the injection site, fever, headache, and malaise, as well as more rare events, such as transverse myelitis, Guillen–Barre syndrome, or platelet activation by vaccine-induced immune thrombocytopenia [14, 17, 18].
Hair loss accompanying COVID-19 infection, as well as its various vaccine strains has been of much concern. No clear understanding of the mechanism behind vaccine-related hair loss had been explained. However, a number of factors were hypothesized [19]. COVID-19-infected individuals had been more prone to microthrombi formation that caused compromise of the vascular circulation within scalp hair follicles [19]. Given its aesthetic impact, hair loss has garnered considerable attention from the general public [20, 21].
Hair Loss (HL), also known as alopecia or baldness, may be classified in several degrees and kinds. Common types include Male- or Female-Pattern Hair Loss (MPHL or FPHL), Alopecia Areata (AA), and a thinning of hair known as Telogen Effluvium (TE). The cause of MPHL is a combination of genetics and male hormones; the cause of FPHL is yet unclear; the cause of AA is autoimmune, and the cause of telogen effluvium is typically a physically or psychologically stressful event [22- 25].
Alopecia Areata (AA) is an autoimmune form of hair loss, usually presenting with patchy hair loss on the scalp. The pathophysiological basis is thought to be the breakdown of the immune privilege of the hair follicle, resulting in increased aggregations of natural killer cells in follicles [26]. External factors such as emotional stress, drugs, and vaccinations have also been implicated. AA can be triggered by viral infections such as influenza [27], cytomegalovirus, and the Epstein-Barr virus [28]. There have been reports in the literature of cases of AA thought to have been triggered by either COVID-19 vaccination or COVID-19 infection [29].
Although alopecia exacerbation following COVID19 vaccinations is rarely reported, it is a frequent concern among patients. Alopecia areata has been observed following routine vaccinations, such as influenza, zoster, and human papilloma virus vaccines. Thus, an association cannot be ignored [30, 31].
Telogen Effluvium (TE) is one of the most popular alopecia in women, provoked by stressful events, trauma, illness, malnutrition, hormonal imbalance, and drugs. The pattern of hair loss in telogen effluvium is diffuse, without scars, and involves less than half of the hair. It occurs 2-3 months after the stressful condition and is occasionally self-limiting. Telogen effluvium could be chronic if it lasts more than 6 months. The patients who suffer from telogen effluvium are anxious and usually worry about their hair. Therefore, telogen effluvium has a dramatic impact on their psychological health and mind [32].
Telogen effluvium–associated hair loss can last up to 6 months after proper treatment, as well as removal of any triggering factors. Persistence of telogen effluvium for more than 6 months implies chronicity of the condition and can be more challenging to treat. In a study, Ammar et al. found that approximately 23.9% of patients had diffuse hair loss for 6 months (acute TE), with the majority commencing one month post-vaccination (45.8%) and 33.2% after the second month [33].
The UK Medicines and Healthcare Products Regulatory Agency has published data on the adverse events reported following COVID-19 vaccination [34]. A total of 154 cases of AA, alopecia totalis (AT, affecting the whole head), or alopecia universalis (AU, affecting the whole body) have been reported; 50% with the BNT162b2 Pfizer/BioNTech (New York, NY), 40% with the ChAdOx1 nCoV‐19 AstraZeneca (Cambridge, UK), and 10% with the mRNA-1273 Moderna (Cambridge, MA) vaccines. Although this may simply be a reflection of the proportion of each vaccine administered in the United Kingdom, a possible association may be present. The US Centers for Disease Control and Prevention Vaccine Adverse Event Reporting System database [35] was searched for reported cases of AA, AT, and AU following COVID-19 vaccination; it showed a total of 126 cases (114 of AA, 1 of AT, and 11 of AU). The vast majority were Pfizer/BioNTech (66%) and Moderna (29%). One must consider that some cases of mild hair loss may not have been reported, meaning that the true number of cases may be higher.
Hair loss may be a disturbing side effect that has a substantial influence on the quality of life of those who suffer from it. Several studies have reported the association between vaccines and hair loss. For instance, a study by Alharbi reported an increase in telogen effluvium in patients who received the COVID-19 vaccine [36]. Another study by Scollan et al. found that hair loss occurred in some individuals who received the Moderna vaccine [37]. These studies have raised questions about the potential link between COVID-19 vaccines and hair loss.
While these studies provide valuable insights, their limitations, such as small sample sizes and lack of statistical analysis, highlight the need for more extensive investigations to fully understand the association between COVID-19 vaccines and hair loss. As a result, a large-scale investigation is required to explore the possible negative effects of COVID-19 vaccinations on hair loss. Research in this area may provide important insights into possible adverse effects of Covid-19 vaccination, particularly their effect on hair loss, which has received little attention in the literature. Understanding the potential side effects of COVID-19 vaccines is essential to ensure that individuals can make informed decisions about vaccination and healthcare providers can better manage any potential adverse events.
The present study aimed to investigate the effect of COVID-19 vaccinations on hair loss, classify the participants based on sociodemographic status and evaluate the correlation between hair loss and comorbidities, such as hypertension, diabetes, cancer, and allergy.
Instruments and Methods
A retrospective cohort analysis was performed on 580 people aged 20 to 72 years who had received the Covid-19 vaccine in Iraqi cities. The study population included 270 males and 310 females. The sociodemographic characteristics and comorbidities were obtained from medical records.
The participants were asked to report hair loss after receiving the vaccine, and individuals who reported hair loss were classified into two categories based on the severity of hair loss, i.e., mild and severe.
To identify potential risk factors for hair loss, machine learning techniques were used to classify individuals based on sociodemographic factors such as age, gender, education level, income, and occupation. After correcting for possible confounders such as comorbidities, vaccine type, and immunization date, a logistic regression analysis was used to assess the Odds Ratio (OR) and 95% Confidence Interval (CI) for hair loss. Additionally, the study evaluated the effect of different COVID-19 vaccines on hair loss by comparing the occurrence of hair loss among individuals who received different vaccines. The study also assessed the association between the timing of vaccination and the occurrence of hair loss.
Findings
This research included 580 people (270 men and 310 women). The participants' average age was 46.2±12.5 years, with a range of 20 to 72 years. The majority of the participants were from cities (Table 1).
Table 1) Socio-demographic characteristics of study participants (numbers in parentheses are percentages)

Out of the total participants, 17.6% reported experiencing hair loss after receiving the COVID-19 vaccine (Table 2). This percentage was higher in females (19.4%) compared to the males (15.2%). There was a significant association between the COVID-19 vaccine and hair loss in both males and females. The odds ratio for developing hair loss after receiving the COVID-19 vaccine was 1.34 (95% CI: 1.04-1.73) for females and 1.12 (95% CI: 0.81-1.54) for males.
Table 2) Frequency distribution of hair loss after receiving the Covid-19 vaccine in women and men (numbers in parentheses are percentages)

The prevalence of hair loss after COVID-19 vaccination was higher for the Moderna vaccine (30.3%) compared to Pfizer-BioNTech (23.1%) and Johnson & Johnson (7.6%; Table 3).
Table 3) Prevalence of hair loss according to the type of covid-19 vaccine in men and women (numbers in parentheses are percentages)

No significant difference was observed between males and females based on comorbidities such as hypertension, diabetes, cancer, and allergy.
Table 4) Prevalence of hair loss according to comorbidity status in men and women (numbers in parentheses are percentages)

Figures 1 and 2 show the ROC curves for the machine learning analysis of hair loss after Covid-19 vaccination in males and females. AUC was 0.73 (95% CI: 0.68-0.77) for females and 0.67 (95% CI: 0.62-0.72) for males.

Figure 1) ROC curves for the machine learning analysis of hair loss after Covid-19 vaccination in females
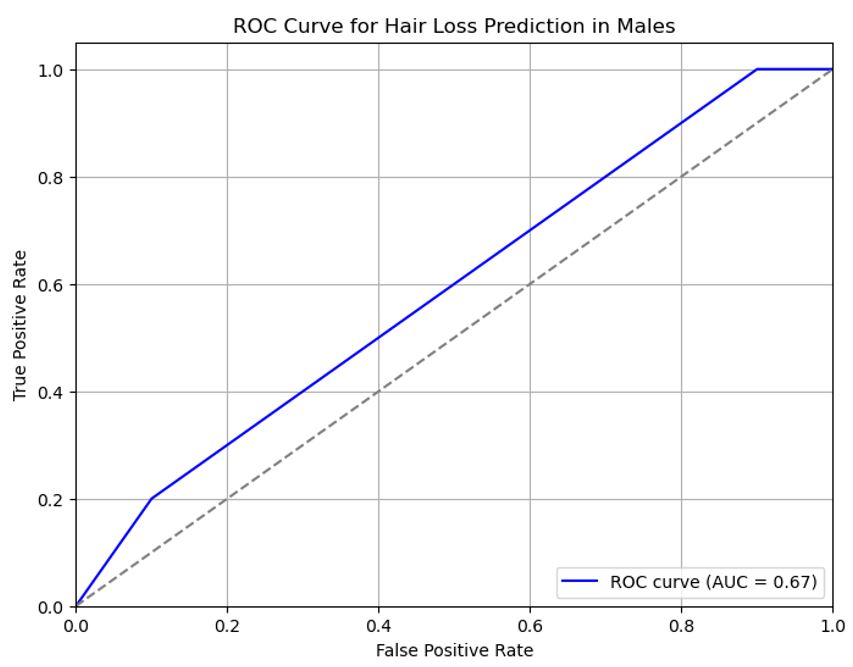
Figure 2) ROC curves for the machine learning analysis of hair loss after Covid-19 vaccination in males
Discussion
Coronavirus disease 2019 (COVID-19) is a major concern worldwide, and various vaccines have been developed and approved for it. However, some immune-related issues of COVID-19 vaccines should be considered and individualized for patients [38].
COVID-19 vaccines have positively changed the course of the pandemic. They entered the market after only one year of the initial trials, which yielded positive results in terms of safety and efficacy. However, after inoculating billions of people in the most extensive vaccination campaign worldwide, mild but common and some rare but potentially fatal adverse events have been reported. Among several self-reported adverse events, hair loss and alopecia have been linked to COVID-19 mRNA or viral vector vaccines [19]. Some of the side effects of the COVID-19 vaccine may be underpinned by molecular mimicry and the production of pathological autoantibodies, causing, for example hair loss [39].
In this study, we investigated the relationship between COVID-19 immunization and hair loss in a group of 270 men and 310 women ranging in age from 20 to 72 years old and categorized by sociodemographic status, with the majority of them residing at cities. Our results showed a significant association between COVID-19 vaccination and hair loss in both males and females.
Several studies have reported adverse effects of COVID-19 vaccination, including hair loss [40, 41]. The mechanism behind this adverse effect is still unclear, but it is hypothesized that the immune response triggered by the vaccine might cause temporary hair loss [42, 43]. More research is needed to examine this mechanism and determine the duration of this negative effect.
Our machine learning analysis showed high accuracy in predicting hair loss in patients who received COVID-19 vaccination. It suggests that machine learning algorithms can be useful in predicting adverse effects of COVID-19 vaccination and can help in identifying patients at risk for such effects.
Our study also found a correlation between comorbidities such as hypertension, diabetes, cancer, and allergy with hair loss after COVID-19 vaccination. This result is consistent with other research that showed individuals with comorbidities were more likely to experience negative side effects from the COVID-19 immunization [44]. More study is needed to examine the processes behind this connection and if individuals with comorbidities should get alternative vaccination formulations or doses.
Hair loss after COVID-19 vaccination has not been widely discussed in the literature; however, a study from Poland demonstrated that only 2.2% of those vaccinated complained of acute hair loss, of which 46.4% and 53.6% having hair loss after the first and second doses, respectively [45].
Ammar et al. assessed dermoscopically the prevalence of hair loss among an Egyptian population following COVID-19 vaccination. They reported prevalence of post-vaccination hair fall that was confirmed by trichoscopy and affected approximately one quarter of participants who received COVID-19 vaccines. Also, they stated that other factors, such as stress and infection, cannot be excluded [33].
Another study from Saudi Arabia demonstrated hair loss after COVID vaccine to be as high as 63.2%, with the majority after the first dose (55.8%) and 44.2% after the second dose [46].
Alharbi’s study that assessed the prevalence of telogen effluvium following COVID 19 vaccination demonstrated that out of 991 participants, 670 (67.6%) reported post-vaccination hair fall. The probable causes of post-vaccination hair fall were vaccine-related in 185 (27.6%) participants, other causes in 326 (48.7%) participants, and unclear in 326 (48.7%) participants [36]. In Alharbi’s study, the majority of participants were females (90.3%). The most common age group was between 21 and 30 years (51.9%). Post-vaccination hair fall was significantly higher among females compared with males (68.5% versus 57.9%, p=0.036) [36]. These findings are in consistent with our results.
In their study, Scollan et al. highlighted that patients with personal or family histories of AA and other autoimmune diseases, particularly thyroid dysfunction, may be at higher risk of hair loss following SARS-CoV-2 vaccination [37].
A case report indicated the possible role of Oxford/AstraZeneca vaccine in causing alopecia areata in genetically preconditioned individuals via mechanisms of immunity [47]. Alfredo Ross et al. described 3 cases of patients who experienced a relapse of alopecia areata after a single dose of the vaccine, 2-3 weeks after vaccination [3]. In the study by Birkett et al., a total of 18 patients were described who developed AA after COVID-19 vaccination, for which AstraZeneca, Moderna, and Pfizer/BioNTech vaccines were used [29].
Arroyo et al. tracked and followed a series of five cases with post-vaccine telogen effluvium and alopecia development in Ecuador. They reported the clinical presentation of two women and three men with the diagnosis of post-vaccine hair loss. All patients received a heterologous vaccination scheme (mRNA and attenuated virus vaccine) with an additional viral vector booster associated with the apparition of telogen effluvium and alopecia universalis between 3 and 17 days after the vaccine was administered. Arroyo et al.'s research supports the hypothesis that there is some association between hair loss and COVID-19 vaccination and expands on the theoretical rationale for the use of mixed vaccination schedules and the Sinovac vaccine [19].
The SARS-CoV-2 virus can make the immune system overactive due to the interaction and molecular mimicry between the virus and self-antigens. Similar to the disease itself, COVID-19 vaccines can increase inflammatory responses [6]. Nuclear Factor Kappa B (NF-κB) activation, which leads to the release of many cytokines, and elevated Interferons (IFN) and Interleukin 6 (IL-6), are linked to AA pathology and might cause hair loss [48]. AZD1222 expresses the S glycoprotein, which stimulates both antibody production and T helper (Th1) cell activation [6] This type of hair loss has also been noticed among recipients of other types of COVID-19 vaccines, such as BNT162b2 (Pfizer–BioNTech) and mRNA-1273 (Moderna) [37]. Furthermore, as Fivenson suggested, the mental stress of the global pandemic may have contributed to some of the cases, as stress has been recognized as a trigger for hair loss [49].
Knowledge about SARS-CoV-2 vaccine safety and benefits is evolving to support decision-making about use of these vaccines. Although benefits of these vaccines greatly overweight risks associated with acquisition of infection, the benefit-risk balance should be communicated to patients. There is currently a lack of clear-cut recommendations about screening for autoimmunity in patients receiving SARS-CoV-2 vaccines, and autoimmunity in this context is multifactorial with multiple modifiers. Due to the growing reports of autoimmunity flares, including AA, healthcare providers should remember to enquire about personal and/or family history of autoimmunity. This would allow for proper patient-centered counselling and enable patients to take informed health decisions in their best interest [50].
Overall, our findings add to the expanding body of research on the negative consequences of COVID-19 immunization and shed light on the utility of machine learning algorithms in anticipating bad effects. These findings can help develop personalized vaccination strategies for patients with different risk profiles.
Our study found that hair loss was a rare but possible side effect of COVID-19 vaccination in both males and females. We were able to identify possible risk factors and sociodemographic features related with this side effect by using machine learning methods. The results suggest that individuals with certain comorbidities, such as hypertension and diabetes, may be at a higher risk for experiencing hair loss after COVID-19 vaccination. Providers of healthcare should be aware of this possible adverse effect and advice patients accordingly. Future studies should investigate the underlying mechanisms of this side effect and further explore its risk factors and long-term consequences.
Conclusion
Hair loss is a rare but possible side effect of COVID-19 vaccination in both males and females, which its prevalence is higher in females than in males. Individuals with certain comorbidities, such as hypertension and diabetes, may be at a higher risk for experiencing hair loss after COVID-19 vaccination.
Acknowledgements: We would like to acknowledge the participants of this study for their time and contribution to the research. We would also like to thank the healthcare providers who assisted in the data collection process.
Ethical Permission: This research project has received ethical approval from the local ethical committees at Al-Zahra Teaching Hospital and Iraqi Private Hospital, Iraq (Code number of Iraqi Health Ministry Approval: 02/2021).
Conflict of Interests: The authors declare no conflict of interests.
Authors’ Contribution: Yousif MG (First Author), Main Researcher (35%); Al-Jumeily D (Second Author), Introduction Writer/Methodologist/Statistical Analyst (25%); Al-Amran FG (Third Author), Methodologist/Statistical Analyst/Discussion Writer (20%); Sadeq AM (Fourth Author), Statistical Analyst/Discussion Writer (10%); Rawaf S (Fifth Author), Introduction Writer/Discussion Writer (10%)
Funding: This study received no particular support from governmental, commercial, or not-profit funding entities.
The novel coronavirus disease 2019 (COVID-19) outbreak was declared a global pandemic by the World Health Organization (WHO) on March 11, 2020 [1]. Over 468 million infections and more than 6 million deaths worldwide have been caused by the Coronavirus disease 2019 (COVID-19) [2]. Various vaccines have been developed and approved for COVID-19 globally. Due to the new variants (e.g., Omicron), the use of additional doses is also being reviewed and approved for prevention [3]. The COVID-19 pandemic has had a severe effect on world health and the economy, and vaccination has been critical in limiting its spread [4, 5].
There is still insufficient information on the immune-related issues of COVID-19 vaccines and whether or not they can cause the flare of autoimmune diseases [6]. The quick development and distribution of COVID-19 vaccination has aided in the reduction of cases and deaths globally [7]. However, while the efficacy and safety of vaccines have been extensively studied, their potential side effects remain a concern [8].
Vaccine-induced adverse events are a concern among both healthy individuals and those with preexisting conditions. The concern is that the safety of any vaccine cannot be easily generalizable to populations who are not routinely included in clinical trials, such as patients with chronic autoimmune and inflammatory disorders [9]. The paucity of data and concerns related to disease exacerbations of these preexisting inflammatory conditions can serve as a source of vaccine hesitancy among patients. Several studies have observed exacerbations of preexisting autoimmune or autoinflammatory disorders in at-risk populations postvaccination [10].
The development of vaccines throughout human history has changed the course of diseases and humanity [11]. The process of vaccine development often requires decades to obtain a marketable vaccine; nevertheless, with the advent of the SARS-CoV-2 pandemic in March 2020, the process was accelerated by both necessity and the amount of resources available for this purpose [12]. While this process has followed the highest standards of safety and control, due to the large number of people vaccinated in all corners of the globe, reports of adverse events are still very common [13, 14]. In this context, by December 2020, the Food and Drug Administration (FDA) authorized the emerging use of the mRNA-based vaccine produced by Pfizer/BioNTech (BNT162b2), followed by Moderna (ARNm-1273), and later the rest of the COVID-19 vaccines, including the viral-vector-based AstraZeneca (ChAdOx1), or the attenuated and inactivated virus vaccine from Sinopharm (BBIBP-CorV) and Sinovac (Sinovac-CoronaVac) [15].
With initial reports on safety and effectivity, and after the biggest vaccination campaign took place all over the planet, adverse events started to appear [15, 16]. Among the most common and expected are pain at the injection site, fever, headache, and malaise, as well as more rare events, such as transverse myelitis, Guillen–Barre syndrome, or platelet activation by vaccine-induced immune thrombocytopenia [14, 17, 18].
Hair loss accompanying COVID-19 infection, as well as its various vaccine strains has been of much concern. No clear understanding of the mechanism behind vaccine-related hair loss had been explained. However, a number of factors were hypothesized [19]. COVID-19-infected individuals had been more prone to microthrombi formation that caused compromise of the vascular circulation within scalp hair follicles [19]. Given its aesthetic impact, hair loss has garnered considerable attention from the general public [20, 21].
Hair Loss (HL), also known as alopecia or baldness, may be classified in several degrees and kinds. Common types include Male- or Female-Pattern Hair Loss (MPHL or FPHL), Alopecia Areata (AA), and a thinning of hair known as Telogen Effluvium (TE). The cause of MPHL is a combination of genetics and male hormones; the cause of FPHL is yet unclear; the cause of AA is autoimmune, and the cause of telogen effluvium is typically a physically or psychologically stressful event [22- 25].
Alopecia Areata (AA) is an autoimmune form of hair loss, usually presenting with patchy hair loss on the scalp. The pathophysiological basis is thought to be the breakdown of the immune privilege of the hair follicle, resulting in increased aggregations of natural killer cells in follicles [26]. External factors such as emotional stress, drugs, and vaccinations have also been implicated. AA can be triggered by viral infections such as influenza [27], cytomegalovirus, and the Epstein-Barr virus [28]. There have been reports in the literature of cases of AA thought to have been triggered by either COVID-19 vaccination or COVID-19 infection [29].
Although alopecia exacerbation following COVID19 vaccinations is rarely reported, it is a frequent concern among patients. Alopecia areata has been observed following routine vaccinations, such as influenza, zoster, and human papilloma virus vaccines. Thus, an association cannot be ignored [30, 31].
Telogen Effluvium (TE) is one of the most popular alopecia in women, provoked by stressful events, trauma, illness, malnutrition, hormonal imbalance, and drugs. The pattern of hair loss in telogen effluvium is diffuse, without scars, and involves less than half of the hair. It occurs 2-3 months after the stressful condition and is occasionally self-limiting. Telogen effluvium could be chronic if it lasts more than 6 months. The patients who suffer from telogen effluvium are anxious and usually worry about their hair. Therefore, telogen effluvium has a dramatic impact on their psychological health and mind [32].
Telogen effluvium–associated hair loss can last up to 6 months after proper treatment, as well as removal of any triggering factors. Persistence of telogen effluvium for more than 6 months implies chronicity of the condition and can be more challenging to treat. In a study, Ammar et al. found that approximately 23.9% of patients had diffuse hair loss for 6 months (acute TE), with the majority commencing one month post-vaccination (45.8%) and 33.2% after the second month [33].
The UK Medicines and Healthcare Products Regulatory Agency has published data on the adverse events reported following COVID-19 vaccination [34]. A total of 154 cases of AA, alopecia totalis (AT, affecting the whole head), or alopecia universalis (AU, affecting the whole body) have been reported; 50% with the BNT162b2 Pfizer/BioNTech (New York, NY), 40% with the ChAdOx1 nCoV‐19 AstraZeneca (Cambridge, UK), and 10% with the mRNA-1273 Moderna (Cambridge, MA) vaccines. Although this may simply be a reflection of the proportion of each vaccine administered in the United Kingdom, a possible association may be present. The US Centers for Disease Control and Prevention Vaccine Adverse Event Reporting System database [35] was searched for reported cases of AA, AT, and AU following COVID-19 vaccination; it showed a total of 126 cases (114 of AA, 1 of AT, and 11 of AU). The vast majority were Pfizer/BioNTech (66%) and Moderna (29%). One must consider that some cases of mild hair loss may not have been reported, meaning that the true number of cases may be higher.
Hair loss may be a disturbing side effect that has a substantial influence on the quality of life of those who suffer from it. Several studies have reported the association between vaccines and hair loss. For instance, a study by Alharbi reported an increase in telogen effluvium in patients who received the COVID-19 vaccine [36]. Another study by Scollan et al. found that hair loss occurred in some individuals who received the Moderna vaccine [37]. These studies have raised questions about the potential link between COVID-19 vaccines and hair loss.
While these studies provide valuable insights, their limitations, such as small sample sizes and lack of statistical analysis, highlight the need for more extensive investigations to fully understand the association between COVID-19 vaccines and hair loss. As a result, a large-scale investigation is required to explore the possible negative effects of COVID-19 vaccinations on hair loss. Research in this area may provide important insights into possible adverse effects of Covid-19 vaccination, particularly their effect on hair loss, which has received little attention in the literature. Understanding the potential side effects of COVID-19 vaccines is essential to ensure that individuals can make informed decisions about vaccination and healthcare providers can better manage any potential adverse events.
The present study aimed to investigate the effect of COVID-19 vaccinations on hair loss, classify the participants based on sociodemographic status and evaluate the correlation between hair loss and comorbidities, such as hypertension, diabetes, cancer, and allergy.
Instruments and Methods
A retrospective cohort analysis was performed on 580 people aged 20 to 72 years who had received the Covid-19 vaccine in Iraqi cities. The study population included 270 males and 310 females. The sociodemographic characteristics and comorbidities were obtained from medical records.
The participants were asked to report hair loss after receiving the vaccine, and individuals who reported hair loss were classified into two categories based on the severity of hair loss, i.e., mild and severe.
To identify potential risk factors for hair loss, machine learning techniques were used to classify individuals based on sociodemographic factors such as age, gender, education level, income, and occupation. After correcting for possible confounders such as comorbidities, vaccine type, and immunization date, a logistic regression analysis was used to assess the Odds Ratio (OR) and 95% Confidence Interval (CI) for hair loss. Additionally, the study evaluated the effect of different COVID-19 vaccines on hair loss by comparing the occurrence of hair loss among individuals who received different vaccines. The study also assessed the association between the timing of vaccination and the occurrence of hair loss.
Findings
This research included 580 people (270 men and 310 women). The participants' average age was 46.2±12.5 years, with a range of 20 to 72 years. The majority of the participants were from cities (Table 1).
Table 1) Socio-demographic characteristics of study participants (numbers in parentheses are percentages)

Out of the total participants, 17.6% reported experiencing hair loss after receiving the COVID-19 vaccine (Table 2). This percentage was higher in females (19.4%) compared to the males (15.2%). There was a significant association between the COVID-19 vaccine and hair loss in both males and females. The odds ratio for developing hair loss after receiving the COVID-19 vaccine was 1.34 (95% CI: 1.04-1.73) for females and 1.12 (95% CI: 0.81-1.54) for males.
Table 2) Frequency distribution of hair loss after receiving the Covid-19 vaccine in women and men (numbers in parentheses are percentages)

The prevalence of hair loss after COVID-19 vaccination was higher for the Moderna vaccine (30.3%) compared to Pfizer-BioNTech (23.1%) and Johnson & Johnson (7.6%; Table 3).
Table 3) Prevalence of hair loss according to the type of covid-19 vaccine in men and women (numbers in parentheses are percentages)

No significant difference was observed between males and females based on comorbidities such as hypertension, diabetes, cancer, and allergy.
Table 4) Prevalence of hair loss according to comorbidity status in men and women (numbers in parentheses are percentages)

Figures 1 and 2 show the ROC curves for the machine learning analysis of hair loss after Covid-19 vaccination in males and females. AUC was 0.73 (95% CI: 0.68-0.77) for females and 0.67 (95% CI: 0.62-0.72) for males.

Figure 1) ROC curves for the machine learning analysis of hair loss after Covid-19 vaccination in females

Figure 2) ROC curves for the machine learning analysis of hair loss after Covid-19 vaccination in males
Discussion
Coronavirus disease 2019 (COVID-19) is a major concern worldwide, and various vaccines have been developed and approved for it. However, some immune-related issues of COVID-19 vaccines should be considered and individualized for patients [38].
COVID-19 vaccines have positively changed the course of the pandemic. They entered the market after only one year of the initial trials, which yielded positive results in terms of safety and efficacy. However, after inoculating billions of people in the most extensive vaccination campaign worldwide, mild but common and some rare but potentially fatal adverse events have been reported. Among several self-reported adverse events, hair loss and alopecia have been linked to COVID-19 mRNA or viral vector vaccines [19]. Some of the side effects of the COVID-19 vaccine may be underpinned by molecular mimicry and the production of pathological autoantibodies, causing, for example hair loss [39].
In this study, we investigated the relationship between COVID-19 immunization and hair loss in a group of 270 men and 310 women ranging in age from 20 to 72 years old and categorized by sociodemographic status, with the majority of them residing at cities. Our results showed a significant association between COVID-19 vaccination and hair loss in both males and females.
Several studies have reported adverse effects of COVID-19 vaccination, including hair loss [40, 41]. The mechanism behind this adverse effect is still unclear, but it is hypothesized that the immune response triggered by the vaccine might cause temporary hair loss [42, 43]. More research is needed to examine this mechanism and determine the duration of this negative effect.
Our machine learning analysis showed high accuracy in predicting hair loss in patients who received COVID-19 vaccination. It suggests that machine learning algorithms can be useful in predicting adverse effects of COVID-19 vaccination and can help in identifying patients at risk for such effects.
Our study also found a correlation between comorbidities such as hypertension, diabetes, cancer, and allergy with hair loss after COVID-19 vaccination. This result is consistent with other research that showed individuals with comorbidities were more likely to experience negative side effects from the COVID-19 immunization [44]. More study is needed to examine the processes behind this connection and if individuals with comorbidities should get alternative vaccination formulations or doses.
Hair loss after COVID-19 vaccination has not been widely discussed in the literature; however, a study from Poland demonstrated that only 2.2% of those vaccinated complained of acute hair loss, of which 46.4% and 53.6% having hair loss after the first and second doses, respectively [45].
Ammar et al. assessed dermoscopically the prevalence of hair loss among an Egyptian population following COVID-19 vaccination. They reported prevalence of post-vaccination hair fall that was confirmed by trichoscopy and affected approximately one quarter of participants who received COVID-19 vaccines. Also, they stated that other factors, such as stress and infection, cannot be excluded [33].
Another study from Saudi Arabia demonstrated hair loss after COVID vaccine to be as high as 63.2%, with the majority after the first dose (55.8%) and 44.2% after the second dose [46].
Alharbi’s study that assessed the prevalence of telogen effluvium following COVID 19 vaccination demonstrated that out of 991 participants, 670 (67.6%) reported post-vaccination hair fall. The probable causes of post-vaccination hair fall were vaccine-related in 185 (27.6%) participants, other causes in 326 (48.7%) participants, and unclear in 326 (48.7%) participants [36]. In Alharbi’s study, the majority of participants were females (90.3%). The most common age group was between 21 and 30 years (51.9%). Post-vaccination hair fall was significantly higher among females compared with males (68.5% versus 57.9%, p=0.036) [36]. These findings are in consistent with our results.
In their study, Scollan et al. highlighted that patients with personal or family histories of AA and other autoimmune diseases, particularly thyroid dysfunction, may be at higher risk of hair loss following SARS-CoV-2 vaccination [37].
A case report indicated the possible role of Oxford/AstraZeneca vaccine in causing alopecia areata in genetically preconditioned individuals via mechanisms of immunity [47]. Alfredo Ross et al. described 3 cases of patients who experienced a relapse of alopecia areata after a single dose of the vaccine, 2-3 weeks after vaccination [3]. In the study by Birkett et al., a total of 18 patients were described who developed AA after COVID-19 vaccination, for which AstraZeneca, Moderna, and Pfizer/BioNTech vaccines were used [29].
Arroyo et al. tracked and followed a series of five cases with post-vaccine telogen effluvium and alopecia development in Ecuador. They reported the clinical presentation of two women and three men with the diagnosis of post-vaccine hair loss. All patients received a heterologous vaccination scheme (mRNA and attenuated virus vaccine) with an additional viral vector booster associated with the apparition of telogen effluvium and alopecia universalis between 3 and 17 days after the vaccine was administered. Arroyo et al.'s research supports the hypothesis that there is some association between hair loss and COVID-19 vaccination and expands on the theoretical rationale for the use of mixed vaccination schedules and the Sinovac vaccine [19].
The SARS-CoV-2 virus can make the immune system overactive due to the interaction and molecular mimicry between the virus and self-antigens. Similar to the disease itself, COVID-19 vaccines can increase inflammatory responses [6]. Nuclear Factor Kappa B (NF-κB) activation, which leads to the release of many cytokines, and elevated Interferons (IFN) and Interleukin 6 (IL-6), are linked to AA pathology and might cause hair loss [48]. AZD1222 expresses the S glycoprotein, which stimulates both antibody production and T helper (Th1) cell activation [6] This type of hair loss has also been noticed among recipients of other types of COVID-19 vaccines, such as BNT162b2 (Pfizer–BioNTech) and mRNA-1273 (Moderna) [37]. Furthermore, as Fivenson suggested, the mental stress of the global pandemic may have contributed to some of the cases, as stress has been recognized as a trigger for hair loss [49].
Knowledge about SARS-CoV-2 vaccine safety and benefits is evolving to support decision-making about use of these vaccines. Although benefits of these vaccines greatly overweight risks associated with acquisition of infection, the benefit-risk balance should be communicated to patients. There is currently a lack of clear-cut recommendations about screening for autoimmunity in patients receiving SARS-CoV-2 vaccines, and autoimmunity in this context is multifactorial with multiple modifiers. Due to the growing reports of autoimmunity flares, including AA, healthcare providers should remember to enquire about personal and/or family history of autoimmunity. This would allow for proper patient-centered counselling and enable patients to take informed health decisions in their best interest [50].
Overall, our findings add to the expanding body of research on the negative consequences of COVID-19 immunization and shed light on the utility of machine learning algorithms in anticipating bad effects. These findings can help develop personalized vaccination strategies for patients with different risk profiles.
Our study found that hair loss was a rare but possible side effect of COVID-19 vaccination in both males and females. We were able to identify possible risk factors and sociodemographic features related with this side effect by using machine learning methods. The results suggest that individuals with certain comorbidities, such as hypertension and diabetes, may be at a higher risk for experiencing hair loss after COVID-19 vaccination. Providers of healthcare should be aware of this possible adverse effect and advice patients accordingly. Future studies should investigate the underlying mechanisms of this side effect and further explore its risk factors and long-term consequences.
Conclusion
Hair loss is a rare but possible side effect of COVID-19 vaccination in both males and females, which its prevalence is higher in females than in males. Individuals with certain comorbidities, such as hypertension and diabetes, may be at a higher risk for experiencing hair loss after COVID-19 vaccination.
Acknowledgements: We would like to acknowledge the participants of this study for their time and contribution to the research. We would also like to thank the healthcare providers who assisted in the data collection process.
Ethical Permission: This research project has received ethical approval from the local ethical committees at Al-Zahra Teaching Hospital and Iraqi Private Hospital, Iraq (Code number of Iraqi Health Ministry Approval: 02/2021).
Conflict of Interests: The authors declare no conflict of interests.
Authors’ Contribution: Yousif MG (First Author), Main Researcher (35%); Al-Jumeily D (Second Author), Introduction Writer/Methodologist/Statistical Analyst (25%); Al-Amran FG (Third Author), Methodologist/Statistical Analyst/Discussion Writer (20%); Sadeq AM (Fourth Author), Statistical Analyst/Discussion Writer (10%); Rawaf S (Fifth Author), Introduction Writer/Discussion Writer (10%)
Funding: This study received no particular support from governmental, commercial, or not-profit funding entities.
Article Type: Original Research |
Subject:
Health Care
Received: 2023/04/23 | Accepted: 2023/07/16 | Published: 2023/08/1
Received: 2023/04/23 | Accepted: 2023/07/16 | Published: 2023/08/1
References
1. Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157-60. [Link]
2. World Health Organization. COVID-19 weekly epidemiological update, edition 84 [Internet]. Geneva: World Health Organization; 2022 [cited 2023 Feb 29]. Available from: https://apps.who.int/iris/handle/10665/352608. [Linkvvv]
3. Rossi A, Magri F, Michelini S, Caro G, Di Fraia M, Fortuna MC, et al. Recurrence of alopecia areata after covid-19 vaccination: A report of three cases in Italy. J Cosmet Dermatol. 2021;20(12):3753-7. [Link] [DOI:10.1111/jocd.14581]
4. Yousif NG, Altimimi AN, Al-amran FG, Adrienne J, Al-Fadhel SM, Hussien SR, et al. Hematological changes among Corona virus-19 patients: a longitudinal study. Syst Rev Pharm. 2020;11(5):862-6. [Link]
5. Yousif MG, Sadeq AM, Alfadhel SM, Al-Amran FG, Al-Jumeily D. The effect of Hematological parameters on pregnancy outcome among pregnant women with Corona Virus -19 infection: a prospective cross-sectional study. J Surv Fisheries Sci. 2023;10(3S):1425-35. [Linkvv]
6. World Health Organization. COVID-19 weekly epidemiological update, edition 58 [Internet]. Geneva: World Health Organization; 2021 [cited 2023 Feb 29]. Available from: https://apps.who.int/iris/handle/10665/345456 [Link]
7. Centers for Disease Control and Prevention. Vaccines for COVID-19 [Internet]. Atlanta, US: Centers for Disease Control and Prevention; 2019 [cited 2023 Feb 24]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html [Link]
8. World Health Organization. Coronavirus disease (COVID-19) [Internet]. Geneva: World Health Organization; 2019 [cited 2023 Feb 24]. Available from: https://www.who.int/health-topics/coronavirus [Link]
9. Geisen UM, Berner DK, Tran F, Sümbül M, Vullriede L, Ciripoi M, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Diss. 2021;80(10):1306-11. [Link] [DOI:10.1136/annrheumdis-2021-220272]
10. Terracina KA, Tan FK. Flare of rheumatoid arthritis after COVID-19 vaccination. Lancet Rheumatol. 2021;3(7):e469-70. [Link] [DOI:10.1016/S2665-9913(21)00108-9]
11. Drummond M, Chevat C, Lothgren M. Do we fully understand the economic value of vaccines? Vaccine. 2007;25(32):5945-57. [Link] [DOI:10.1016/j.vaccine.2007.04.070]
12. Ortiz-Prado E, Simbaña-Rivera K, Gómez-Barreno L, Rubio-Neira M, Guaman LP, Kyriakidis NC, et al. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the coronavirus disease 2019 (COVID-19), a comprehensive literature review. Diagn Microbiol Infect Dis. 2020;98(1):115094. [Link] [DOI:10.1016/j.diagmicrobio.2020.115094]
13. Krause PR, Gruber MF. Emergency use authorization of COVID vaccines-safety and efficacy follow-up considerations. N Engl J Med. 2020;383(19):e107. [Link] [DOI:10.1056/NEJMp2031373]
14. Tatar M, Faraji MR, Montazeri Shoorekchali J, Pagán JA, Wilson FA. The role of good governance in the race for global vaccination during the COVID-19 pandemic. Sci Rep. 2021:11:22440. [Link] [DOI:10.1038/s41598-021-01831-0]
15. Lee GM, Romero JR, Bell BP. Postapproval vaccine safety surveillance for COVID-19 vaccines in the US. JAMA. 2020;324(19):1937-8. [Link] [DOI:10.1001/jama.2020.19692]
16. Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. 2021;194:245-51. [Link] [DOI:10.1016/j.puhe.2021.02.025]
17. Kaur RJ, Dutta S, Bhardwaj P, Charan J, Dhingra S, Mitra P, et al. Adverse events reported from COVID-19 vaccine trials: a systematic review. Indian J Clin Biochem. 2021;36(4):427-39. [Link] [DOI:10.1007/s12291-021-00968-z]
18. Ortiz-Prado E, Izquierdo-Condoy JS, Fernandez-Naranjo R, Simbaña-Rivera K, Vásconez-González J, Naranjo EPL, et al. A comparative analysis of a self-reported adverse events analysis after receiving one of the available SARS-CoV-2 vaccine schemes in Ecuador. Vaccines. Vaccines (Basel). 2022;10(7):1047. [Link] [DOI:10.3390/vaccines10071047]
19. Arroyo JH, Izquierdo-Condoy JS, Ortiz-Prado E. A case series and literature review of telogen effluvium and alopecia universalis after the administration of a heterologous COVID-19 vaccine scheme. Vaccines (Basel). 2023;11(2):444. [Link] [DOI:10.3390/vaccines11020444]
20. Esen-Salman K, Akın-Çakıcı Ö, Kardeş S, Salman A. Public interest in dermatologic symptoms, conditions, treatments, and procedures during the COVID-19 pandemic: insights from Google trends. Dermatol Ther. 2021;34(2):e14895. [Link] [DOI:10.1111/dth.14895]
21. Gunderson J, Mitchell D, Reid K, Jordan M. COVID-19 information-seeking and prevention behaviors in Florida, April 2020. Prev Chronic Dis. 2021;18:E17. [Link] [DOI:10.5888/pcd18.200575]
22. Gentile P, Garcovich S. The effectiveness of low-level light/laser therapy on hair loss. Facial Plast Surg Aesthet Med. 2021. [Link] [DOI:10.1089/fpsam.2021.0151]
23. Gentile P, Calabrese C, De Angelis B, Dionisi L, Pizzicannella J, Kothari A, et al. Impact of the different preparation methods to obtain autologous non-activated platelet-rich plasma (A-PRP) and activated platelet-rich plasma (AA-PRP) in plastic surgery: wound healing and hair regrowth evaluation. Int. J Mol Sci. 2020:21(2):431. [Link] [DOI:10.3390/ijms21020431]
24. Gentile P, Garcovich S, Bielli A, Scioli MG, Orlandi A, Cervelli V. The effect of platelet-rich plasma in hair regrowth: A randomized placebo-controlled trial. Stem Cells Transl. Med. 2015;4(11):1317-23. [Link] [DOI:10.5966/sctm.2015-0107]
25. Gentile P, Cole JP, Cole MA, Garcovich S, Bielli A, Scioli MG, et al. Evaluation of not-activated and activated PRP in hair loss treatment: role of growth factor and cytokine concentrations obtained by different collection systems. Int J Mol Sci. 2017;18(2):408. [Link] [DOI:10.3390/ijms18020408]
26. Pratt C, King L, Messenger A, Christiano A, Sundberg J. Alopecia areata. Nat Rev Dis Primers. 2017;3(1):17011. [Link] [DOI:10.1038/nrdp.2017.11]
27. Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546. [Link] [DOI:10.1155/2013/348546]
28. Rajabi F, Drake LA, Senna MM, Rezaei N. Alopecia areata: a review of disease pathogenesis. Br J Dermatol. 2018;179(5):1033-48. [Link] [DOI:10.1111/bjd.16808]
29. Birkett L, Singh P, Mosahebi A, Dhar S. Possible associations between alopecia areata and COVID-19 vaccination and infection. Aesthet Surg J. 2022;42(11):NP699-702. [Link] [DOI:10.1093/asj/sjac165]
30. Chu CH, Cheng YP, Chan JYL. Alopecia areata after vaccination: recurrence with rechallenge. Pediatr Dermatol. 2016;33(3):e218-9. [Link] [DOI:10.1111/pde.12849]
31. Wise RP, Kiminyo KP, Salive ME. Hair loss after routine immunizations. JAMA. 1997;278(14):1176-8. [Link] [DOI:10.1001/jama.1997.03550140068042]
32. Rivetti N, Barruscotti S. Management of telogen effluvium during the COVID-19 emergency: Psychological implications. Dermatol Ther. 2020;33(4):e13648. [Link] [DOI:10.1111/dth.13648]
33. Ammar AM, Ibrahim IS, Mohamed AN, Elsaie ML. Dermoscopy-assisted prevalence of hair loss after COVID-19 vaccination among an Egyptian population: a cross-sectional study. Ir J Med Sci. 2023;1971:3. [Link] [DOI:10.1007/s11845-023-03493-5]
34. UK Government. Coronavirus (COVID-19) vaccines adverse reactions [Internet]. London: UK Government; 2022 [cited 2023 Feb 10]. Available from: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions [Link]
35. Vaccine Adverse Events Reporting System. VAERS data [Internet]. US: VAERS; 2022 [cited 2023 Feb 15]. Available from: https://vaers.hhs.gov/data.html [Link]
36. Alharbi M. Telogen effluvium after COVID-19 vaccination among public in Saudi Arabia. J Family Med Prim Care. 2022;11(10): 6056-60. [Link] [DOI:10.4103/jfmpc.jfmpc_377_22]
37. Scollan ME, Breneman A, Kinariwalla N, Soliman Y, Youssef S, Bordone LA, et al. Alopecia areata after SARS-CoV-2 vaccination. JAAD Case Rep. 2022;20:1-5. [Link] [DOI:10.1016/j.jdcr.2021.11.023]
38. Ganjei Z, Yazdan Panah M, Rahmati R, Zari Meidani F, Mosavi A. COVID-19 vaccination and alopecia areata: a case report and literature review. Clin Case Rep. 2022;10(9):e6039. [Link] [DOI:10.1002/ccr3.6039]
39. Essam R, Ehab R, Al-Razzaz R, Khater MW, Moustafa EA. Alopecia areata after ChAdOx1 nCoV-19vaccine (Oxford/AstraZeneca): a potential triggering factor? J Cosmet Dermatol. 2021;20(12):3727-9. [Link] [DOI:10.1111/jocd.14459]
40. Blumenthal KG, Freeman EE, Saff RR, Robinson LB, Wolfson AR, Foreman RK, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384(13):1273-7. [Link] [DOI:10.1056/NEJMc2102131]
41. He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672-5. [Link] [DOI:10.1038/s41591-020-0869-5]
42. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of Coronavirus (COVID-19) [Internet]. StatPearls Publishing; 2022 [cited 2023 Feb 24]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554776/ [Link]
43. Murugan S, Assi S, Alatrany A, Jayabalan M, Liatsis P, Mustafina J, et al. Consumer behavior prediction during Covid-19 pandemic conditions using sentiment analytics. In The International Conference on Data Science and Emerging Technologies. Singapore: Springer Nature Singapore; 2022 Dec 20. pp. 209-21. [Link] [DOI:10.1007/978-981-99-0741-0_15]
44. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10(7):806-13. [Link] [DOI:10.1002/alr.22579]
45. Jęśkowiak I, Wiatrak B, Grosman-Dziewiszek P, Szeląg A. The incidence and severity of post-vaccination reactions after vaccination against COVID-19. Vaccines (Basel). 2021;9(5):502. [Link] [DOI:10.3390/vaccines9050502]
46. Alsahli W, Almulhim Y, Issa NT. Cross-sectional study to determine the prevalence of telogen effluvium among patients experiencing COVID-19 infection or vaccination in Saudi Arabia and Arabic countries. Eur J Mol Clin Med. 2022;9(7):4663-74. [Link]
47. Jiang Y, Shi Q, Huang Y, Li J, Xie H, Liu F. Relationship between the exercise and severityof androgenic alopecia. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 28 lipiec 2021;46(7):725-30. [Link]
48. Ganjei Z, Dana HF, Ebrahimi-Dehkordi S, Alidoust F, Bahmani K. Methotrexate as a safe immunosuppressive agent during the COVID-19 pandemic. Int Immunopharmacol. 2021;101(Pt B):108324. [Link] [DOI:10.1016/j.intimp.2021.108324]
49. Fivenson D. COVID-19: association with rapidly progressive forms of alopecia areata. Int J Dermatol. 2020;60(1):127. [Link] [DOI:10.1111/ijd.15317]
50. Hani Abdalla H, Ebrahim E. Alopecia areata universalis precipitated by SARS-CoV-2 Vaccine: a case report and narrative review. Cureus. 2022;14(8):e27953. [Link] [DOI:10.7759/cureus.27953]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







