Volume 10, Issue 1 (2022)
Health Educ Health Promot 2022, 10(1): 201-206 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khodaparast Zavareh M, Abdollah Zadeh Arpanahi M, Gholami Fesharaki M, Sadeghi Ghahrodi M, Mousavi S, Ghazale A, et al . The Risk Factors for Mortality of Adult Inpatients with Covid-19 in Tehran, Iran: A Retrospective Cohort Study. Health Educ Health Promot 2022; 10 (1) :201-206
URL: http://hehp.modares.ac.ir/article-5-57448-en.html
URL: http://hehp.modares.ac.ir/article-5-57448-en.html
M. Khodaparast Zavareh1, M. Abdollah Zadeh Arpanahi2, M. Gholami Fesharaki3, M. Sadeghi Ghahrodi *4, S.V. Mousavi2, A.H. Ghazale4, L. Khedmat5, A. Asadollah6
1- “Department of Echocardiography, School of Medicine” and “Atherosclerosis Research Center”, Baqiyatallah University of Medical Sciences, Tehran, Iran
2- Student Research Committee, Baqiyatallah University of Medical Sciences, Tehran, Iran
3- Biostatistics Department, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
4- “Department of Echocardiography, School of Medicine” and “Department of Cardiology, School of Medicine”, Baqiyatallah University of Medical Sciences, Tehran, Iran
5- Health Management Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran
6- Faculty of Medicine, Tehran Medical Sciences Branch, Islamic Azad University, Tehran, Iran
2- Student Research Committee, Baqiyatallah University of Medical Sciences, Tehran, Iran
3- Biostatistics Department, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
4- “Department of Echocardiography, School of Medicine” and “Department of Cardiology, School of Medicine”, Baqiyatallah University of Medical Sciences, Tehran, Iran
5- Health Management Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran
6- Faculty of Medicine, Tehran Medical Sciences Branch, Islamic Azad University, Tehran, Iran
Full-Text [PDF 580 kb]
(4212 Downloads)
| Abstract (HTML) (1803 Views)
Full-Text: (465 Views)
Introduction
In late 2019 began a wave of respiratory diseases in Wuhan (China) On March 11, 2020, according to the World Health Organization, the COVID-19 pandemic was announced [1].
According to the World Health Organization report until March 2022, more than 444 and 6 million people had been infected and died respectively due to COVID-19 diseases around the world [2]. The Molaei et al. study showed that Iran’s Excess Mortality Rate (EMR) during the COVID-19 pandemic was 36% [1]. This is a high EMR indicating the unknown risk factors of mortality due to COVID-19. Previous studies showed that cardiovascular disease, hypertension, congestive heart failure (CHF), chronic kidney disease, and cancer were associated with a significantly greater risk of mortality from COVID-19 [2-6]. Other research also has been shown that extreme COVID-19 disease is closely associated with fever, cough, dyspnea, pneumonia, and computed tomography findings, any opacity in the ground glass, lymphocytopenia, elevated C-reactive protein (CRP), elevated aminotransferase alanine, elevated aminotransferase aspartate, older age, and male sex [4, 7, 8].
Death is primarily caused by respiratory or heart failure [8]. In Iran, mortality rates for hospitalized COVID-19 have been reported in the range of 8 to 21% [9-14]. Until now, there is no effective drug for this newly-emerged pandemic disease. The widely current use of medications like antiviral therapies including Kaletra (Lopinavir–Ritonavir), Remdesivir, Oseltamivir, Ribavirin, and Sofosbuvir [15] or using Immunomodulators such as Chlorine and Hydroxychloride [16] and anti-inflammatory therapies such as corticosteroids [17] or combination of them [18] has been proposed.
Due to the lack of sufficient knowledge in this field, and considering the importance of mortality management in the control of COVID-19 disease, this study was performed to investigate the risk factors for mortality in COVID-19 adult inpatients.
Material & Methods
This retrospective cohort study was carried out on 393 COVID-19 patients hospitalized in a main military general hospital (Tehran, Iran) from 4 May to 20 June 2020. Baqiyatallah is a general military referral hospital with more than 3000 COVID-19 patients admitted in one month. In this study for sampling, a list of patients was prepared from electronic medical records, and then 459 patients were selected using systematic random sampling by R software, and laboratory data, clinical, treatment, and demographic data were collected and compared between survivors and non-survivors. The required sample size was estimated at 393, based on the prevalence formula
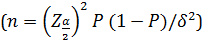
with P=50 % (maximum variability), precision (δ) =5%, α=5% and a dropout rate of 10% (α=5%, β=10%). In this study, 1-hospitalized from 4 May to 20 June 2020, 2-16≤Age≤ 100, 3-approved diagnosis of COVID-19 utilizing positive Real-time polymerase chain reaction (RT-PCR) of throat-swab specimens or the chest CT scans [19] included in this study. For data analysis, patients with high missing information in medical records were excluded (Figure 1).
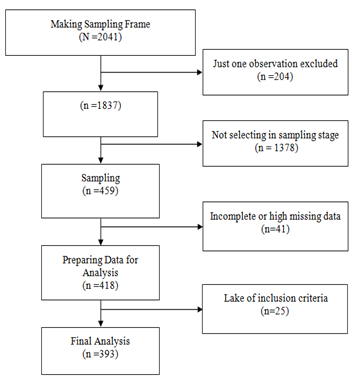
Figure 1) Consort follow the diagram
The demographics, i.e., age, sex, weight, and height in addition to symptoms (e.g. fever temperature≥37.3c, myalgia, diarrhea, chest pain, headache, dyspnea, orthopnea, palpitation), comorbidity (e.g., hypertension, diabetes, CHF, coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), hyperkeratosis lenticularis perstans (HLP), cerebral palsy (CP), Acute kidney injury (AKI)), Laboratory (white blood cell (WBC), CP, CRP, lymphocyte, hemoglobin, platelet count (PLT), lactate dehydrogenase (LDH), Sodium, Potassium, troponin, Procalcitonin, D-dimer), Imaging features (Lesion type, Focality and CT scan score described by Pan et al. [20] in addition vital sign (respiratory rate (RR), pulse rate (PR), blood pressure (BP), and oxygen saturation ) were evaluated. Both BP and PR levels
were evaluated electronically through a bedside monitor (BSM-5132; Nihon-Kohden, Tokyo, Japan). The body temperature was determined with a digital thermometer (Omega Engineering Ltd., Manchester, UK) with a precision of ±0.1°C. The RR was also counted for 1 minute at the bedside.
The statistical analysis was carried out using Windows software R version 3.1.2 (R Project for Statistical Computing, Vienna, Austria) at a significant level of p<0.05. Qualitative and quantitative variables were reported by frequency (percent) and median (±IQR), respectively. The distribution normality of quantitative variables was checked by the Kolmogorov Smirnov test. The Mann–Whitney U test or T-test, and Chi-square or Fisher’s exact test were used to compare quantitative and qualitative variables between two groups, respectively.
Findings
423 patients were hospitalized and randomly selected in Baqiyatallah hospital with COVID-19 from 4 May to 20 June 2020. After excluding, 393 patients have been evaluated. Among 393 patients 37 (9.4%) with 95% confidence interval (6.7% to 12.7%) died during hospitalization. The median age of the 393 patients was as 58 years (IQR 49–67), ranging from 15 years to 97 years, and most patients were female (Table 1). The most common symptoms on admission were cough, dyspnea, myalgia, fever, headache, and diarrhea (Table1). The most common comorbidity on admission was Hypertension, Diabetes, and HLP.
The Patient laboratory findings on admission time according to the two groups are presented in Table 2. The result of this table showed that Lymphocytopenia occurred in 67 (17%) patients. leukopenia, leukocytosis, and anemia occurred in 53 (14%), 56 (14%), and 85 (22%) respectively.
The most lesion type was ground-glass +consolidation, ground-glass, and ground-glass crazy paving (Table 3). More results (Table 1 to 3) showed that comorbidity like hypertension and CHF, sign and vital signs like dyspnea, RR>24, and Oxygen saturation also laboratory variables like WBC, Lymphocyte, CRP, CR, ESR, LDH, Sodium, troponin, procalcitonin in addition lesion type shown the significant relationship with patients death.
The most drugs used with patients were Azithromycin 307 (78%), Naproxen 310 (79%), Hydroxychloroquine 245 (62%), Kaltra 202 (51%), Ceftriaxone 172 (44%) and Preddisolon 110 (28%). Using the drug including Kaltra, Vancomycin, Ribavirin, Meropenem, Levofloxacin, and Methyiprednisolon increased the risk of death but using the drug Azithromycin, Hydroxychloroquine and Naproxen decreased death risk. More results also showed that ARDS, AKI, and intubation are more common in Non-survivor rather than survivor patients (Table 4).
Table 1) Patient demographic and comorbidity findings on admission time according to the two groups

Table 2) Patient laboratory findings on admission time according to the two groups
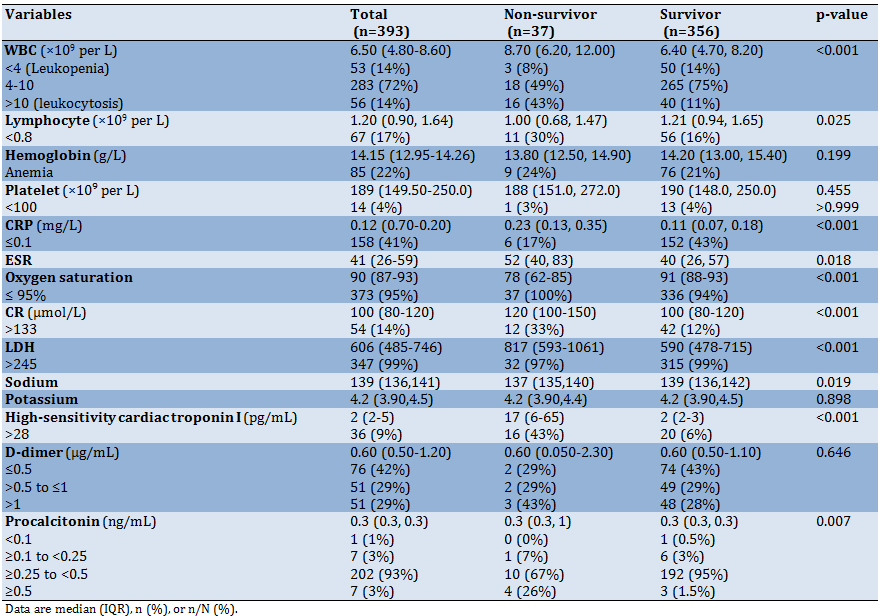
Table 3) Patient imaging features on admission time according to the two groups

Table 4) Treatments and outcomes according to the survivor and non-survivor

Discussion
In recent year, the COVID-19 pandemic has been had a notable impact on the mortality rate and socio-economic costs of the health system in different countries of the world [6].
Contradictory research on this viral disease continues to discuss the challenges of achieving successful prevention, control, and treatment methods.
Due to the lack of sufficient knowledge in this field, and considering the importance of mortality management in the control of COVID-19 disease, this study was performed to investigate the risk factors for mortality in COVID-19 adult inpatients. The result of this study showed that among 393 patients 37 (9.4%) with 95% confidence interval (6.7% to 12.7%) died during hospitalization. this mortality rate is lower than studies [3,9-13, 21], and upper than study done by Shahriarirad et al. [14] in Iran. this rate is lower than COVID-19 hospitalization death in US (p=20%) , Europe (p=23%) and China (p=11%) [22]. This means that despite US sanctions, which have led to major problems in the supply of medicine and equipment in Iran [23], the performance of the Iranian medical staff is good.
More results of this study showed that two comorbidities (hypertension and CHF) in addition 4 vital sign (RR, fever, dyspnea and oxygen saturation) are significantly related to COVID-19 death. Previous studies showed that cardiovascular disease, CHF were associated with a significantly greater risk of mortality from COVID-19 [2-4].
More results also showed that admission laboratory variable like WBC, Lymphocyte, CRP, CR, ESR, LDH, Sodium, troponin, procalcitonin shown the significant relationship with patient’s death. This results is in the line of studies [24-29].
Further result showed like studies done by Wu et al. [29] and Nascimento et al. [25] showed that lesion type is significantly related with patients death.
The findings of this study showed that the use of drug including Kaltra, Vancomycin, Ribavirin, Meropenem, Levofloxasin, and Methyiprednisolon increased the risk of death but use of drug like Azithromycin, Hydroxychloroquin and Naproxen decrease risk of death in COVID-19 hospitalized patients.
The reason for this can be justified that an early upper respiratory tract shedding of the virus has been confirmed in asymptomatic and paucisymptomatic patients during the very first days of symptoms [30], Therefore, just antiviral regimens and handling of virus load may lost their
opportunities over time in symptomatic patients, where patients in this study were not in early phase. Anti-inflammatory drugs have been recommended for the treatment of pulmonary lesions and improvement of clinical outcomes among some selected patients due to their effectiveness in controlling cytokine storms [31].
The limitation of this study was lack of recording for some clinical and laboratory findings due to incomplete patient profiles in the electronic medical records of the hospitals. Therefore, suggestions made in this study were based only on the data that were available to us.
Conclusion
According to the risk factors identified in this study, patients with a higher chance of death can be identified and the necessary treatment measures can be taken to reduce the risk of mortality in these patients.
Acknowledgements: The authors gratefully acknowledge financial support from Baqiyatallah University of Medical Sciences and wish to thank all the personnel, especially the staff of Baqiyatallah hospital, for their cooperation throughout the study.
Ethical permissions: The research procedure was entirely in line with the Baqiyatallah University of Medical Sciences' Human Ethics Committee and implemented with an ethical code IR.BMSU.REC.1399.046.
Conflicts of interests: The authors declare that he has no conflicts of interest. Study is a part of PhD dissertation.
Authors contribution: Khodaparast Zavareh M (First author), Main Researcher (20%); Abdollah Zadeh Arpanahi M (Second author), Assistant Researcher (10%); Gholami Fesharaki M (Third author), Statistical Analyst (20%); Sadeghi Ghahrodi M (Forth author), Main Researcher (20%); Mousavi SV (Fifth author), Assistant Researcher (5%); Ghazale AH (Sixth author), Assistant Researcher (5%); Khedmat L (Seventh author), Discussion Writer (10%); Asadollah A (Eighth author), Introduction Writer (10%)
Funding/Support: This study was funded by Baqiyatallah University of Medical Sciences (Grant number 1399.046).
In late 2019 began a wave of respiratory diseases in Wuhan (China) On March 11, 2020, according to the World Health Organization, the COVID-19 pandemic was announced [1].
According to the World Health Organization report until March 2022, more than 444 and 6 million people had been infected and died respectively due to COVID-19 diseases around the world [2]. The Molaei et al. study showed that Iran’s Excess Mortality Rate (EMR) during the COVID-19 pandemic was 36% [1]. This is a high EMR indicating the unknown risk factors of mortality due to COVID-19. Previous studies showed that cardiovascular disease, hypertension, congestive heart failure (CHF), chronic kidney disease, and cancer were associated with a significantly greater risk of mortality from COVID-19 [2-6]. Other research also has been shown that extreme COVID-19 disease is closely associated with fever, cough, dyspnea, pneumonia, and computed tomography findings, any opacity in the ground glass, lymphocytopenia, elevated C-reactive protein (CRP), elevated aminotransferase alanine, elevated aminotransferase aspartate, older age, and male sex [4, 7, 8].
Death is primarily caused by respiratory or heart failure [8]. In Iran, mortality rates for hospitalized COVID-19 have been reported in the range of 8 to 21% [9-14]. Until now, there is no effective drug for this newly-emerged pandemic disease. The widely current use of medications like antiviral therapies including Kaletra (Lopinavir–Ritonavir), Remdesivir, Oseltamivir, Ribavirin, and Sofosbuvir [15] or using Immunomodulators such as Chlorine and Hydroxychloride [16] and anti-inflammatory therapies such as corticosteroids [17] or combination of them [18] has been proposed.
Due to the lack of sufficient knowledge in this field, and considering the importance of mortality management in the control of COVID-19 disease, this study was performed to investigate the risk factors for mortality in COVID-19 adult inpatients.
Material & Methods
This retrospective cohort study was carried out on 393 COVID-19 patients hospitalized in a main military general hospital (Tehran, Iran) from 4 May to 20 June 2020. Baqiyatallah is a general military referral hospital with more than 3000 COVID-19 patients admitted in one month. In this study for sampling, a list of patients was prepared from electronic medical records, and then 459 patients were selected using systematic random sampling by R software, and laboratory data, clinical, treatment, and demographic data were collected and compared between survivors and non-survivors. The required sample size was estimated at 393, based on the prevalence formula
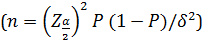
with P=50 % (maximum variability), precision (δ) =5%, α=5% and a dropout rate of 10% (α=5%, β=10%). In this study, 1-hospitalized from 4 May to 20 June 2020, 2-16≤Age≤ 100, 3-approved diagnosis of COVID-19 utilizing positive Real-time polymerase chain reaction (RT-PCR) of throat-swab specimens or the chest CT scans [19] included in this study. For data analysis, patients with high missing information in medical records were excluded (Figure 1).

Figure 1) Consort follow the diagram
The demographics, i.e., age, sex, weight, and height in addition to symptoms (e.g. fever temperature≥37.3c, myalgia, diarrhea, chest pain, headache, dyspnea, orthopnea, palpitation), comorbidity (e.g., hypertension, diabetes, CHF, coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), hyperkeratosis lenticularis perstans (HLP), cerebral palsy (CP), Acute kidney injury (AKI)), Laboratory (white blood cell (WBC), CP, CRP, lymphocyte, hemoglobin, platelet count (PLT), lactate dehydrogenase (LDH), Sodium, Potassium, troponin, Procalcitonin, D-dimer), Imaging features (Lesion type, Focality and CT scan score described by Pan et al. [20] in addition vital sign (respiratory rate (RR), pulse rate (PR), blood pressure (BP), and oxygen saturation ) were evaluated. Both BP and PR levels
were evaluated electronically through a bedside monitor (BSM-5132; Nihon-Kohden, Tokyo, Japan). The body temperature was determined with a digital thermometer (Omega Engineering Ltd., Manchester, UK) with a precision of ±0.1°C. The RR was also counted for 1 minute at the bedside.
The statistical analysis was carried out using Windows software R version 3.1.2 (R Project for Statistical Computing, Vienna, Austria) at a significant level of p<0.05. Qualitative and quantitative variables were reported by frequency (percent) and median (±IQR), respectively. The distribution normality of quantitative variables was checked by the Kolmogorov Smirnov test. The Mann–Whitney U test or T-test, and Chi-square or Fisher’s exact test were used to compare quantitative and qualitative variables between two groups, respectively.
Findings
423 patients were hospitalized and randomly selected in Baqiyatallah hospital with COVID-19 from 4 May to 20 June 2020. After excluding, 393 patients have been evaluated. Among 393 patients 37 (9.4%) with 95% confidence interval (6.7% to 12.7%) died during hospitalization. The median age of the 393 patients was as 58 years (IQR 49–67), ranging from 15 years to 97 years, and most patients were female (Table 1). The most common symptoms on admission were cough, dyspnea, myalgia, fever, headache, and diarrhea (Table1). The most common comorbidity on admission was Hypertension, Diabetes, and HLP.
The Patient laboratory findings on admission time according to the two groups are presented in Table 2. The result of this table showed that Lymphocytopenia occurred in 67 (17%) patients. leukopenia, leukocytosis, and anemia occurred in 53 (14%), 56 (14%), and 85 (22%) respectively.
The most lesion type was ground-glass +consolidation, ground-glass, and ground-glass crazy paving (Table 3). More results (Table 1 to 3) showed that comorbidity like hypertension and CHF, sign and vital signs like dyspnea, RR>24, and Oxygen saturation also laboratory variables like WBC, Lymphocyte, CRP, CR, ESR, LDH, Sodium, troponin, procalcitonin in addition lesion type shown the significant relationship with patients death.
The most drugs used with patients were Azithromycin 307 (78%), Naproxen 310 (79%), Hydroxychloroquine 245 (62%), Kaltra 202 (51%), Ceftriaxone 172 (44%) and Preddisolon 110 (28%). Using the drug including Kaltra, Vancomycin, Ribavirin, Meropenem, Levofloxacin, and Methyiprednisolon increased the risk of death but using the drug Azithromycin, Hydroxychloroquine and Naproxen decreased death risk. More results also showed that ARDS, AKI, and intubation are more common in Non-survivor rather than survivor patients (Table 4).
Table 1) Patient demographic and comorbidity findings on admission time according to the two groups

Table 2) Patient laboratory findings on admission time according to the two groups

Table 3) Patient imaging features on admission time according to the two groups

Table 4) Treatments and outcomes according to the survivor and non-survivor

Discussion
In recent year, the COVID-19 pandemic has been had a notable impact on the mortality rate and socio-economic costs of the health system in different countries of the world [6].
Contradictory research on this viral disease continues to discuss the challenges of achieving successful prevention, control, and treatment methods.
Due to the lack of sufficient knowledge in this field, and considering the importance of mortality management in the control of COVID-19 disease, this study was performed to investigate the risk factors for mortality in COVID-19 adult inpatients. The result of this study showed that among 393 patients 37 (9.4%) with 95% confidence interval (6.7% to 12.7%) died during hospitalization. this mortality rate is lower than studies [3,9-13, 21], and upper than study done by Shahriarirad et al. [14] in Iran. this rate is lower than COVID-19 hospitalization death in US (p=20%) , Europe (p=23%) and China (p=11%) [22]. This means that despite US sanctions, which have led to major problems in the supply of medicine and equipment in Iran [23], the performance of the Iranian medical staff is good.
More results of this study showed that two comorbidities (hypertension and CHF) in addition 4 vital sign (RR, fever, dyspnea and oxygen saturation) are significantly related to COVID-19 death. Previous studies showed that cardiovascular disease, CHF were associated with a significantly greater risk of mortality from COVID-19 [2-4].
More results also showed that admission laboratory variable like WBC, Lymphocyte, CRP, CR, ESR, LDH, Sodium, troponin, procalcitonin shown the significant relationship with patient’s death. This results is in the line of studies [24-29].
Further result showed like studies done by Wu et al. [29] and Nascimento et al. [25] showed that lesion type is significantly related with patients death.
The findings of this study showed that the use of drug including Kaltra, Vancomycin, Ribavirin, Meropenem, Levofloxasin, and Methyiprednisolon increased the risk of death but use of drug like Azithromycin, Hydroxychloroquin and Naproxen decrease risk of death in COVID-19 hospitalized patients.
The reason for this can be justified that an early upper respiratory tract shedding of the virus has been confirmed in asymptomatic and paucisymptomatic patients during the very first days of symptoms [30], Therefore, just antiviral regimens and handling of virus load may lost their
opportunities over time in symptomatic patients, where patients in this study were not in early phase. Anti-inflammatory drugs have been recommended for the treatment of pulmonary lesions and improvement of clinical outcomes among some selected patients due to their effectiveness in controlling cytokine storms [31].
The limitation of this study was lack of recording for some clinical and laboratory findings due to incomplete patient profiles in the electronic medical records of the hospitals. Therefore, suggestions made in this study were based only on the data that were available to us.
Conclusion
According to the risk factors identified in this study, patients with a higher chance of death can be identified and the necessary treatment measures can be taken to reduce the risk of mortality in these patients.
Acknowledgements: The authors gratefully acknowledge financial support from Baqiyatallah University of Medical Sciences and wish to thank all the personnel, especially the staff of Baqiyatallah hospital, for their cooperation throughout the study.
Ethical permissions: The research procedure was entirely in line with the Baqiyatallah University of Medical Sciences' Human Ethics Committee and implemented with an ethical code IR.BMSU.REC.1399.046.
Conflicts of interests: The authors declare that he has no conflicts of interest. Study is a part of PhD dissertation.
Authors contribution: Khodaparast Zavareh M (First author), Main Researcher (20%); Abdollah Zadeh Arpanahi M (Second author), Assistant Researcher (10%); Gholami Fesharaki M (Third author), Statistical Analyst (20%); Sadeghi Ghahrodi M (Forth author), Main Researcher (20%); Mousavi SV (Fifth author), Assistant Researcher (5%); Ghazale AH (Sixth author), Assistant Researcher (5%); Khedmat L (Seventh author), Discussion Writer (10%); Asadollah A (Eighth author), Introduction Writer (10%)
Funding/Support: This study was funded by Baqiyatallah University of Medical Sciences (Grant number 1399.046).
Article Type: Original Research |
Subject:
Social Health
Received: 2021/11/27 | Accepted: 2022/03/15 | Published: 2022/04/10
Received: 2021/11/27 | Accepted: 2022/03/15 | Published: 2022/04/10
References
1. Molaei A, Fesharaki M G. The geographic distribution of excess mortality rate due to covid-19 in Iranian population: an ecological study. Iranian Red Cres Med J. 2021;23(11). [Link] [DOI:10.21203/rs.3.rs-122870/v1]
2. Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PloS One. 2020;15(8):e0238215. [Link] [DOI:10.1371/journal.pone.0238215]
3. Nikpouraghdam M, Jalali Farahani A, Alishiri G, Heydari S, Ebrahimnia M, Samadinia H, et al. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in Iran: a single center study. J Clin Virol. 2020;127:104378. [Link] [DOI:10.1016/j.jcv.2020.104378]
4. Lu L, Zhong W, Bian Z, Li Z, Zhang K, Liang B, et al. A comparison of mortality-related risk factors of COVID-19, SARS, and MERS: A systematic review and meta-analysis. J Infect. 2020;81(4):e18-25. [Link] [DOI:10.1016/j.jinf.2020.07.002]
5. Sadeghi Ghahrodi M, Mousavi SV, Dadjou Y, Khedmat L, Abdollah Zadeh Arpanahi M, Jafari R, et al. COVID-19 prognosis in patients with/without a history of ACEI/ARB consumption. Iran Heart J. 2022;23(1):129-39. [Link]
6. Najafi A, Ghanei M, Janbabaei G, Velayati AA, Saadat SH, Jamaati H, et al. Real clinical practice and therapeutic management following COVID-19 crisis in two hospitals in Iran: a statistical and conceptual view. Tanaffos. 2020;19(2):112-21. [Link]
7. Adeli M, Fesharaki M G. Evaluation of COVID-19 treatment outcomes in a military hospital and its comparison with a nonmilitary hospital. J Mil Med. 2021;23(8):675-83. [Persian] [Link]
8. Keeley P, Buchanan D, Carolan C, Pivodic L, Tavabie S, Noble S. Symptom burden and clinical profile of COVID-19 deaths: a rapid systematic review and evidence summary. BMJ Support Palliat Care. 2020;10:381-4. [Link] [DOI:10.1136/bmjspcare-2020-002368]
9. Rastad H, Karim H, Ejtahed HS, Tajbakhsh R, Noorisepehr M, Babaei M, et al. Risk and predictors of in-hospital mortality from COVID-19 in patients with diabetes and cardiovascular disease. Diabet Metab Syndr. 2020;12:57. [Link] [DOI:10.1186/s13098-020-00565-9]
10. Javanian M, Bayani M, Shokri M, Sadeghi-Haddad-Zavareh M, Babazadeh A, et al. Clinical and laboratory findings from patients with COVID-19 pneumonia in Babol North of Iran: a retrospective cohort study. Rom J Intern Med. 2020;58(3):161-7. [Link] [DOI:10.2478/rjim-2020-0013]
11. Homayounieh F, Zhang EW, Babaei R, Karimi Mobin H, Sharifian M, Mohseni I, et al. Clinical and imaging features predict mortality in COVID-19 infection in Iran. PloS One. 2020;15(9):e0239519. [Link] [DOI:10.1371/journal.pone.0239519]
12. Zali A, Gholamzadeh S, Mohammadi G, Azizmohammad Looha M, Akrami F, Zarean E, et al. Baseline characteristics and associated factors of mortality in Covid-19 patients; an analysis of 16000 cases in Tehran, Iran. Arch Acad Emerg Med. 2020;8(1):e70. [Link]
13. Allameh SF, Nemati S, Ghalehtaki R, Mohammadnejad E, Aghili SM, Khajavirad N, et al. Clinical characteristics and outcomes of 905 Covid-19 patients admitted to Imam Khomeini Hospital Complex in the capital city of Tehran, Iran. Arch Iran Med. 2020;23(11):766-75. [Link] [DOI:10.34172/aim.2020.102]
14. Shahriarirad R, Khodamoradi Z, Erfani A, Hosseinpour H, Ranjbar K, Emami Y, et al. Epidemiological and clinical features of 2019 novel coronavirus diseases (COVID-19) in the South of Iran. BMC Infect Dis. 2020;20: 427. [Link] [DOI:10.1186/s12879-020-05128-x]
15. Mitjà O, Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet. 2020;8(5):e639-40. [Link] [DOI:10.1016/S2214-109X(20)30114-5]
16. Sahu P, Galhotra A, Raj U, et al. A study of self-reported health problems of the people living near railway tracks in Raipur city. J Fam Med Primary Care. 2020;9(2):740-4. [Link] [DOI:10.4103/jfmpc.jfmpc_1029_19]
17. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473-5. [Link] [DOI:10.1016/S0140-6736(20)30317-2]
18. Ghazale AH, Saloo S, Banadkooki A MD, et al. Evaluation of the effect of combination therapy on treatment of Covid-19: a cohort study. Iran Red Crescent Med J. 2021;23(6). [Link]
19. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;20(4):425-34. [Link] [DOI:10.1016/S1473-3099(20)30086-4]
20. Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes at chest ct during recovery from coronavirus disease 2019 (COVID-19). Radiology. 2020;295(3):715-21. [Link] [DOI:10.1148/radiol.2020200370]
21. Malekpour Alamdari N, Afaghi S, Rahimi FS, Esmaeili Tarki F, Tavana S, Zali A, et al. Mortality risk factors among hospitalized covid-19 patients in a major referral center in Iran. Tohoku J Exp Med. 2020;252(1):73-84. [Link] [DOI:10.1620/tjem.252.73]
22. Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PloS One. 2020;15(12):e0243191. [Link] [DOI:10.1371/journal.pone.0243191]
23. Abdoli A. Iran, sanctions, and the COVID-19 crisis. J Med Econ. 2020;30(12):1461-5. [Link] [DOI:10.1080/13696998.2020.1856855]
24. Ali H, Daoud A, Mohamed MM, Abdul Salim S, Yessayan L, Baharani J, et al. Survival rate in acute kidney injury superimposed COVID-19 patients: a systematic review and meta-analysis. Renal Fail. 2020;42(1):393-7. [Link] [DOI:10.1080/0886022X.2020.1756323]
25. Borges do Nascimento IJ, von Groote TC, O'Mathúna DP, Abdulazeem HM, Henderson C, Jayarajah U, et al. Clinical, laboratory and radiological characteristics and outcomes of novel coronavirus (SARS-CoV-2) infection in humans: A systematic review and series of meta-analyses. PloS One. 2020;15(9):e0239235. [Link] [DOI:10.1371/journal.pone.0239235]
26. Simadibrata DM, Lubis AM. D-dimer levels on admission and all-cause mortality risk in COVID-19 patients: a meta-analysis. Epidemiol Infect. 2020;148:e202. [Link] [DOI:10.1017/S0950268820002022]
27. Ghahramani S, Tabrizi R, Lankarani KB, Kashani SMA, Rezaei Sh, Zeidi N, et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. 2020;25:30. [Link] [DOI:10.1186/s40001-020-00432-3]
28. Alnor A, Sandberg MB, Gils C, Vinholt PJ. Laboratory tests and outcome for patients with coronavirus disease 2019: a systematic review and meta-analysis. J Appl Lab Med. 2020;5(5):1038-49. [Link] [DOI:10.1093/jalm/jfaa098]
29. Wu X, Liu L, Jiao J, Yang L, Zhu B, Li X. Characterisation of clinical, laboratory and imaging factors related to mild vs. severe covid-19 infection: a systematic review and meta-analysis. Ann Med. 2020;52(7):334-44. [Link] [DOI:10.1080/07853890.2020.1802061]
30. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N En J Med. 2020;382:970-1 [Link] [DOI:10.1056/NEJMc2001468]
31. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970-5. [Link] [DOI:10.1073/pnas.2005615117]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





